Preparation method of flurbiprofen axetil
The technology of flurbiprofen axetil and flurbiprofen axetil is applied in the field of preparation of pharmaceutical grade flurbiprofen axetil, and can solve the problems of complicated preparation operation, unfavorable unification of quality standards of preparation raw materials and product, increase of preparation production cost and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
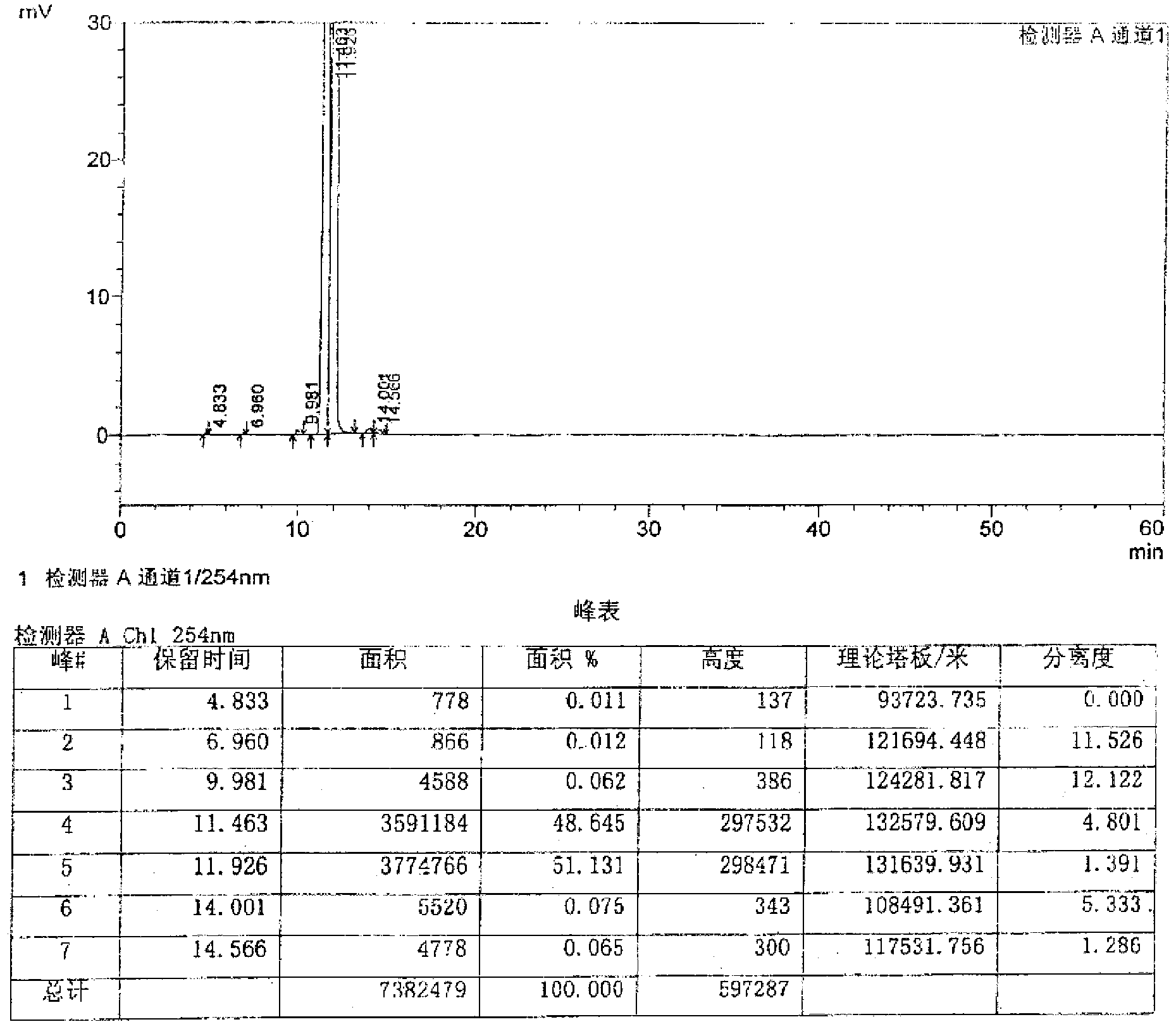
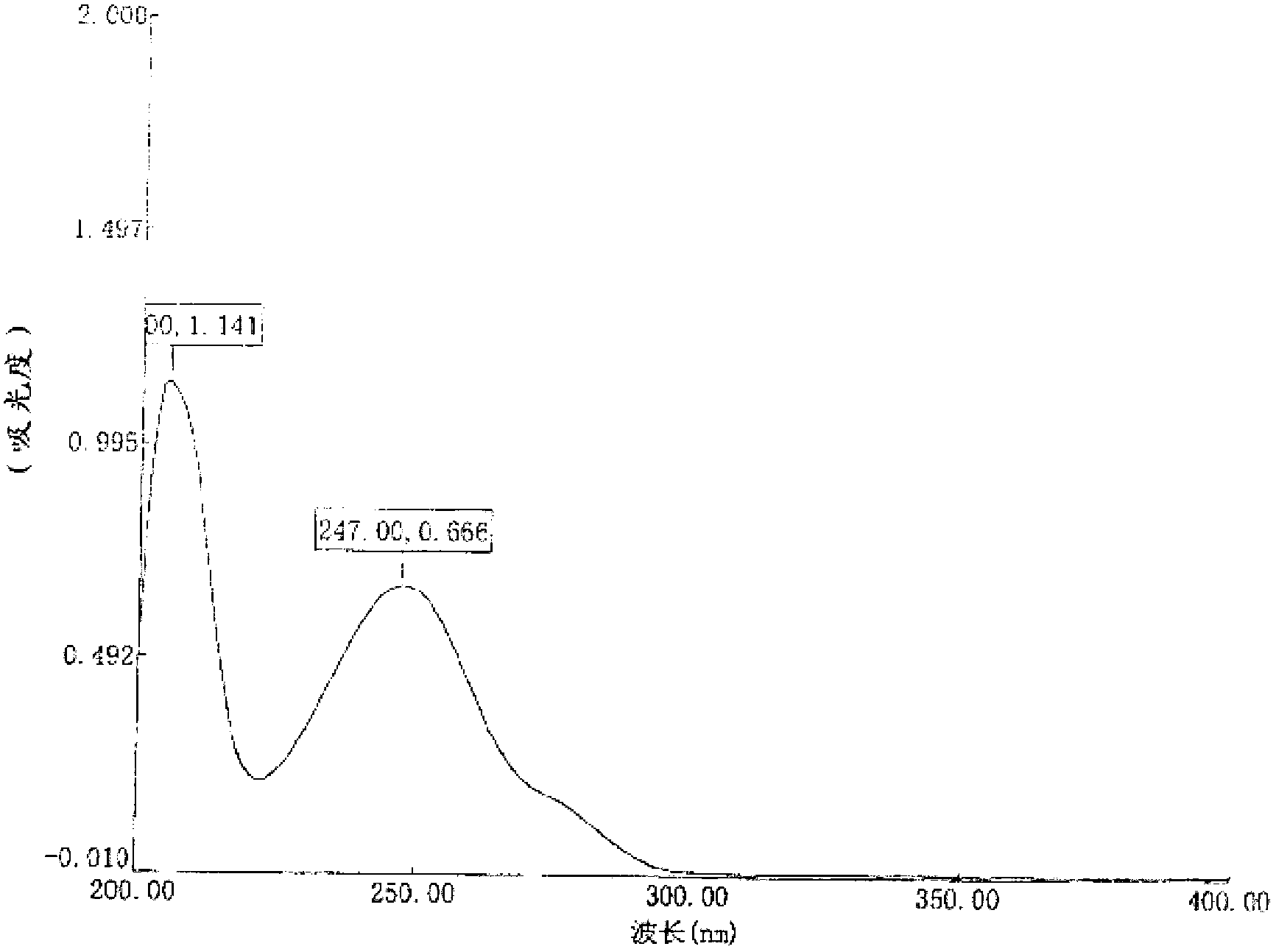
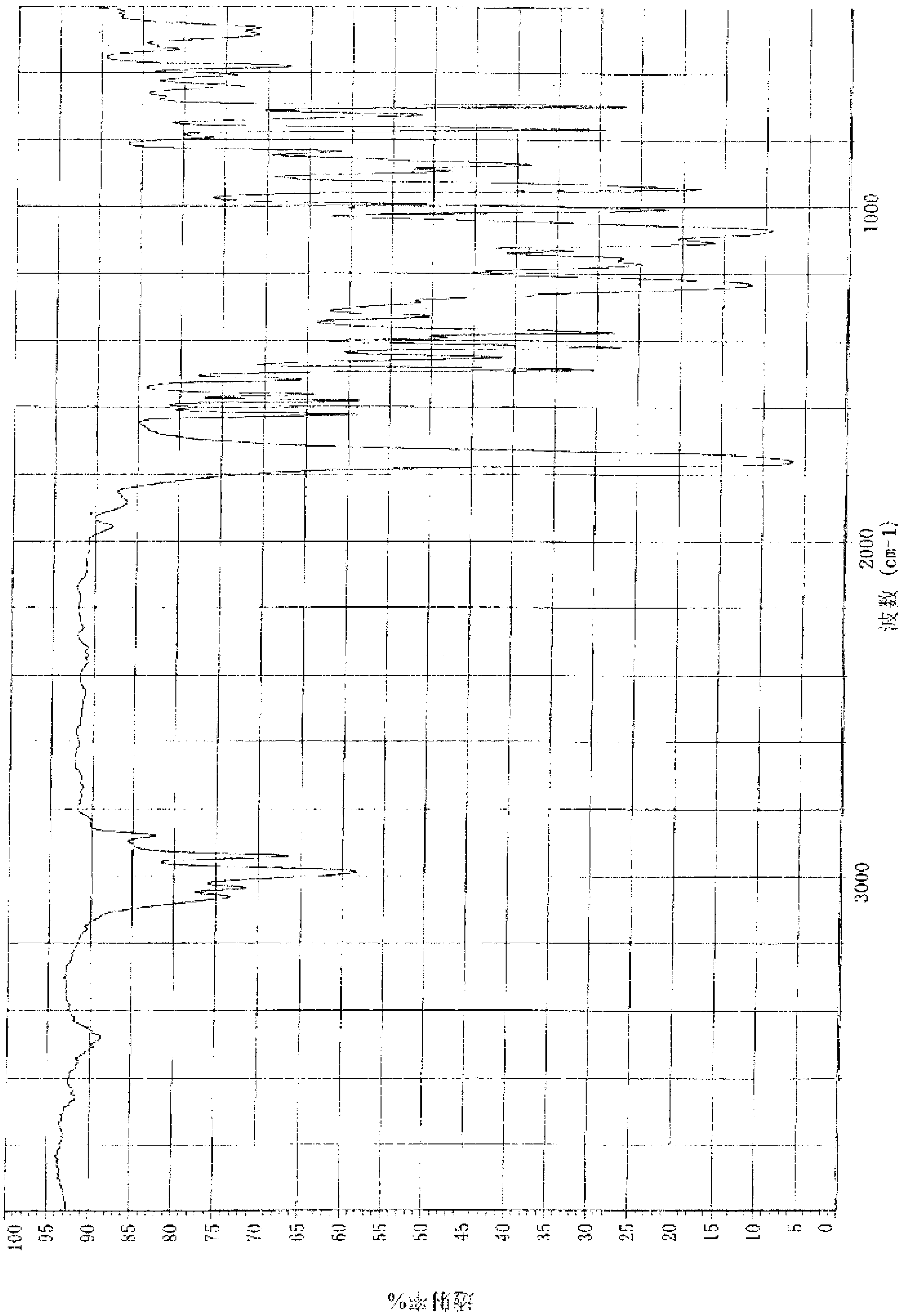
Image
Examples
preparation example Construction
[0020] The invention provides a kind of preparation method of flurbiprofen axetil, comprising the following steps:
[0021] (a) reacting flurbiprofen and bromoethyl acetate in the presence of a catalyst and a solvent;
[0022] (b) washing the product of the reaction to separate the oil;
[0023] (c) Distill the oil under reduced pressure and collect fractions at 173-175°C / 0.8mmHg;
[0024] (d) dissolving the collected fractions in an organic solvent, washing and drying; and
[0025] (e) Removing the organic solvent.
[0026] Flurbiprofen axetil of the present invention refers to (±) 2-(2-fluoro-4-biphenyl)propionic acid-1-acetoxy ethyl ester, and its structural formula is as follows:
[0027]
[0028] In one embodiment of the present invention, the method further comprises the steps of dissolving the separated oil in an organic solvent after step (b), washing (such as washing with water), drying, and removing the organic solvent (b-1 ).
[0029] In one embodiment of th...
Embodiment 1
[0085] Add 12.2g (0.05mol) flurbiprofen (purchased from Kangning Chemical Co., Ltd., Sanmen County, Zhejiang Province) and about 100ml of newly distilled acetone in the reaction flask, mix, add 5.9g (0.07mol) sodium acetate; The reaction mixture was cooled to 5-10°C with ice water, and 11.7g (0.07mol) of bromoethyl acetate (purchased from Quanxi Chemical Factory, Huixian City, Henan Province) was added dropwise. The reaction was stirred for 5h.
[0086] After the reaction was completed, the insoluble matter was filtered off, and about 20 ml of 2.5% (w / v) sodium carbonate aqueous solution was added to the filtrate, and the oily substance in the lower layer was separated after standing. The oil in the lower layer was dissolved in about 100ml of n-hexane, washed with about 30ml of water, and dried by adding anhydrous magnesium sulfate; the desiccant was filtered off, and the n-hexane was evaporated under reduced pressure.
[0087] The obtained product was distilled under reduced...
Embodiment 2
[0091] Add 12.2g (0.05mol) flurbiprofen (purchased from Kangning Chemical Co., Ltd., Sanmen County, Zhejiang Province) and about 100ml of newly distilled ethyl acetate in the reaction flask, mix well, add 7.6g (0.02mol) dodecyl Sodium Phosphate Hydrate. The reaction mixture was cooled to 5-10°C with ice water, and 11.7g (0.07mol) of bromoethyl acetate (purchased from Quanxi Chemical Factory, Huixian City, Henan Province) was added dropwise. The reaction was stirred for 6h.
[0092] After the reaction was completed, the insolubles were filtered off, and about 15ml of 4% (w / v) sodium carbonate aqueous solution was added to the filtrate, and the lower layer oil was separated after standing. The oil in the lower layer was dissolved in about 100ml of n-hexane, washed with about 30ml of water, and dried by adding anhydrous magnesium sulfate; the desiccant was filtered off, and the n-hexane was evaporated under reduced pressure.
[0093] The obtained product was distilled under red...
PUM
| Property | Measurement | Unit |
|---|---|---|
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com