Flurbiprofen axetil eye nano-emulsion in-situ gel preparation and preparation method thereof
A technology of in situ gel and nanoemulsion, applied in polymer solution containing nanoemulsion, preparation of flurbiprofen axetil ophthalmic nanoemulsion-in situ gel preparation, flurbiprofen axetil ophthalmic nanoemulsion- In the field of in situ gel preparations, the effect of prolonging the residence time, easy to use and easy for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
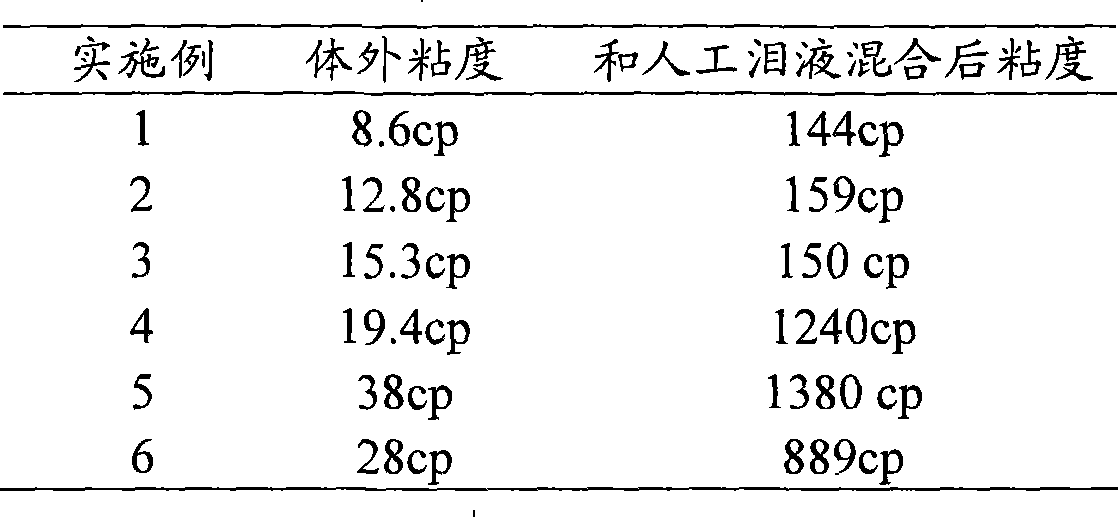
[0031] Table 1 embodiment 1~6 flurbiprofen axetil nanoemulsion-in situ gel preparation composition and process parameter
[0032] Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 flurbiprofen axetil 100mg 300mg 500mg 400mg 200mg 400mg
[0033] Example 1 Example 2 Example 3 Example 4 Example 5 Example 6 Osmolality regulation
[0034] PI: is the polydispersity coefficient of the particle size, which is measured by a particle size analyzer.
Embodiment 2
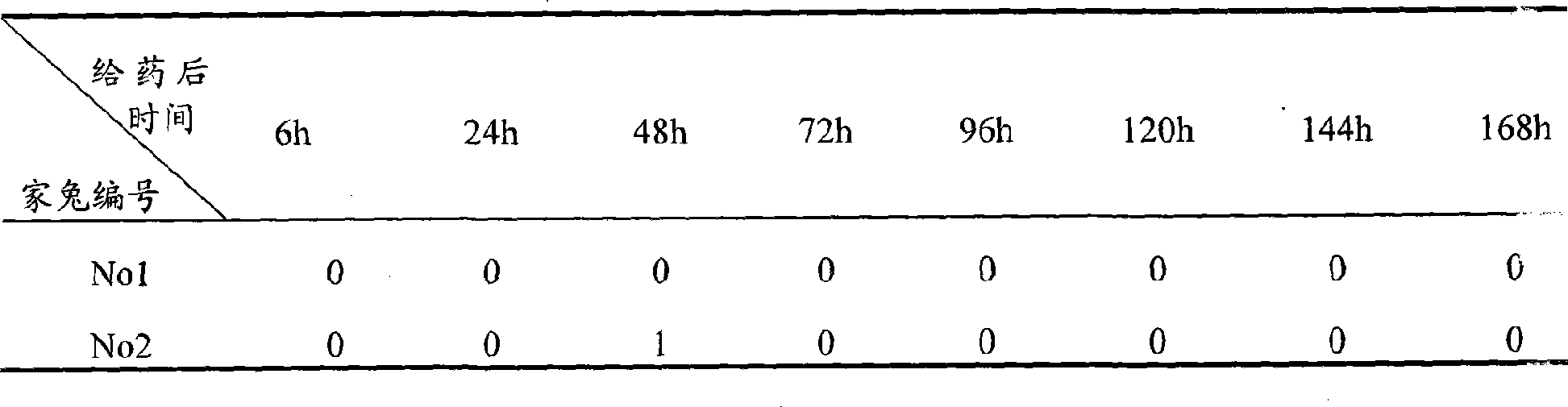
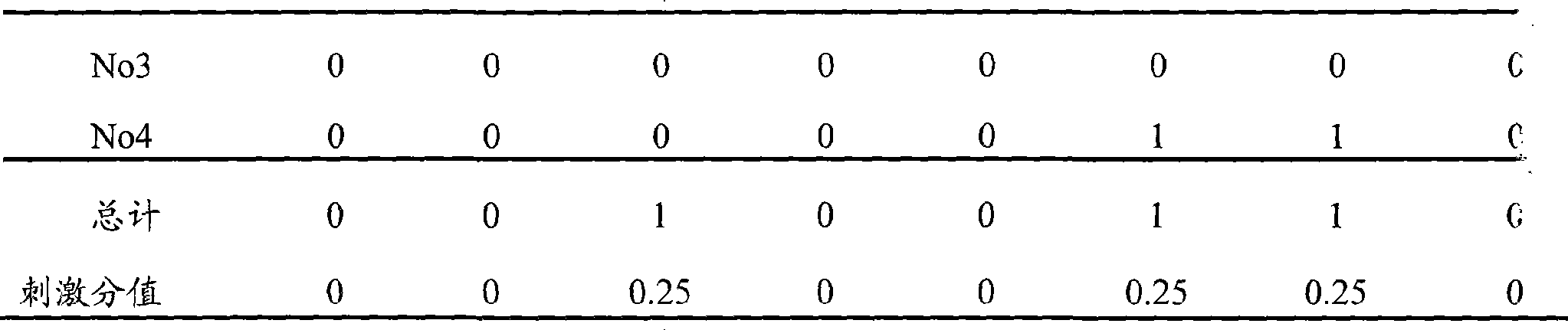
[0039] Test Example 2 Ophthalmic Safety Test of Flurbiprofen Axetil Ophthalmic Nanoemulsion-In Situ Gel Preparation
[0040] 1. Test materials and conditions:
[0041] Test drug: the flurbiprofen axetil ophthalmic nanoemulsion-in situ gel preparation prepared in Example 1, each administration dose is 100 μl;
[0042] Experimental animals: 4 clean New Zealand white rabbits, half male and half male, weighing 2-3 kg (purchased from Shanghai Heping Special Animal and Plant Breeding Farm);
[0043] Animal feeding environment: Room temperature: 20°C
[0044] Humidity: 30%-70%
[0045] Lighting: artificial light, 12 hours daylight, 12 hours dark
[0046] 2. Test method:
[0047] The rabbit's conjunctival sac was gently pulled open, and 100 μl of the test drug was dropped into the conjunctival sac of the right eye, and normal saline was given to the left side as a control. After administration, the eyes are passively closed for 5-10 seconds (the movement...
Embodiment 3
[0053] Test Example 3 Ocular retention evaluation of flurbiprofen axetil ophthalmic nanoemulsion-in situ gel preparation
[0054] 1. Test materials and conditions:
[0055] Experimental animals: 4 clean New Zealand white rabbits, half male and half male, weighing 2-3 kg (purchased from Shanghai Heping Special Animal and Plant Breeding Farm);
[0056] Animal feeding environment: Room temperature: 20°C
[0057] Humidity: 30%-70%
[0058] Lighting: artificial light, 12 hours daylight, 12 hours dark
[0059] Test drug:
[0060] (1), ethyl rhodamine B-labeled flurbiprofen axetil ophthalmic nanoemulsion-in situ gel preparation
[0061] According to the preparation method of Example 1, 100 mg of flurbiprofen axetil, 1 g of soybean lecithin and 0.1 g of ethyl rhodamine B were dissolved in 5 g of soybean oil, and mixed uniformly at 60 ° C to make an oil phase; 20 g of propylene glycol was dissolved in 800 ml Stir and dissolve in purified water at 60°C t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com