A kind of stable flurbiprofen axetil pharmaceutical composition
A technology of flurbiprofen axetil and composition, which is applied in the field of stability of flurbiprofen axetil and its emulsification stability, can solve problems such as increasing potential safety hazards, and achieve the effects of prolonging shelf life, excellent stability, and reducing degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
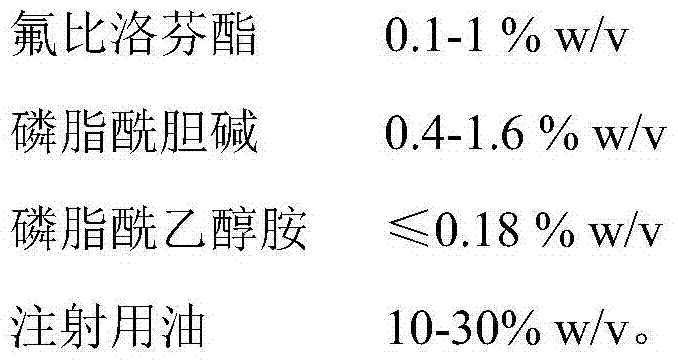
[0064]
[0065] The general steps for emulsion preparation are described below:
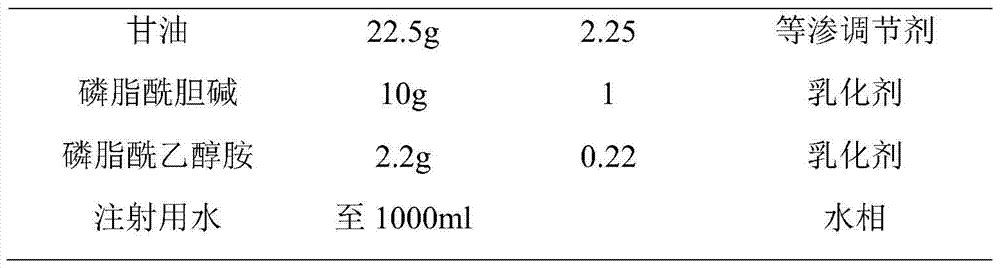
[0066] (1) Take water for injection, heat it to 65°C, add glycerin to dissolve it, and use it as the water phase;
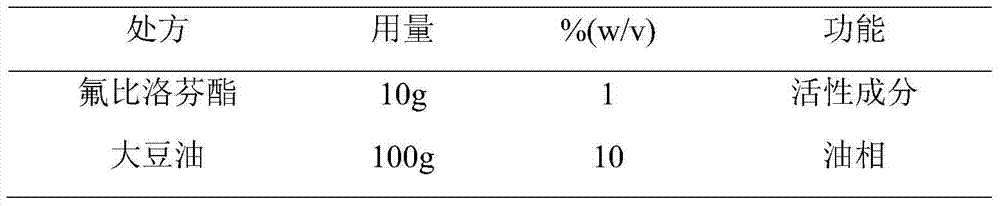
[0067] (2) Take soybean oil, heat it to 65°C, add phosphatidylcholine, phosphatidylethanolamine and flurbiprofen axetil, stir and dissolve, and use it as the oil phase;
[0068] (3) Under high-speed shearing, add the oil phase into the water phase at 65°C, the high-speed shearing speed is 10000rpm, and the time is 10min to form colostrum;
[0069] (4) Disodium hydrogen phosphate citrate buffer solution (the molar ratio of disodium hydrogen phosphate to citric acid is 4:1) to adjust the pH value of the colostrum to 6.0-6.5, and add water for injection to the full amount;
[0070] (5) Transfer the colostrum to a high-pressure homogenizer for emulsification, the homogenization pressure is 1000 bar, and 3 cycles;
[0071] (6) Filtration: filter the essence milk through a 0.45 μm mi...
Embodiment 2
[0074]
[0075] The general steps for emulsion preparation are described below:
[0076] (1) Take water for injection, heat it to 65°C, add glycerin to dissolve it, and use it as the water phase;
[0077] (2) Take soybean oil, heat it to 65°C, add phosphatidylcholine, phosphatidylethanolamine and flurbiprofen axetil, stir and dissolve, and use it as the oil phase;
[0078] (3) Under high-speed shearing, add the oil phase into the water phase at 65°C, the high-speed shearing speed is 10000rpm, and the time is 10min to form colostrum;
[0079] (4) Disodium hydrogen phosphate citrate buffer solution (the molar ratio of disodium hydrogen phosphate to citric acid is 4:1) to adjust the pH value of the colostrum to 6.0-6.5, and add water for injection to the full amount;
[0080] (5) Transfer the colostrum to a high-pressure homogenizer for emulsification, the homogenization pressure is 1000 bar, and 3 cycles;
[0081] (6) Filtration: filter the essence milk through a 0.45 μm mi...
Embodiment 3
[0084]
[0085] The general steps for emulsion preparation are described below:
[0086] (1) Take water for injection, heat it to 65°C, add glycerin to dissolve it, and use it as the water phase;
[0087] (2) Take soybean oil, heat it to 65°C, add phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol and flurbiprofen axetil, stir to dissolve, and use it as the oil phase;
[0088] (3) Under high-speed shearing, add the oil phase into the water phase at 65°C, the high-speed shearing speed is 10000rpm, and the time is 10min to form colostrum;
[0089] (4) Disodium hydrogen phosphate citrate buffer solution (the molar ratio of disodium hydrogen phosphate to citric acid is 4:1) to adjust the pH value of the colostrum to 6.0-6.5, and add water for injection to the full amount;
[0090] (5) Transfer the colostrum to a high-pressure homogenizer for emulsification, the homogenization pressure is 1000 bar, and 3 cycles;
[0091] (6) Filtration: filter the essence milk...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com