Preparation methods of flurbiprofen axetil compound
A technology of flurbiprofen axetil and flurbiprofen, which is applied in the field of preparation of flurbiprofen axetil compounds, can solve the problems of complicated process and high cost, and achieve the effects of simple operation, low production cost and easy acquisition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The invention discloses a preparation method of flurbiprofen ester compounds, the method comprising the following steps:
[0032] 1) Put flurbiprofen and acid catalyst in a molar ratio of 1: (0.02~0.4) in a reactor containing an organic solvent, heat up to 20~150°C, and then drop esters, esters The addition amount of substance is 0.9~8 times of flurbiprofen molar weight, and constant temperature reaction is to the end, obtains reaction system; Among them, the ester substances are added slowly, the dropping time is controlled within 3~36h, and the constant temperature reaction is completed until the reaction system is obtained.
[0033] 2) Concentrate the reaction system by rotary evaporation under reduced pressure, which can remove the solvent in the reaction system, then add ethyl acetate and water with a volume ratio of 1: (0.5~2) to dissolve, and let stand The layers were separated to give an organic phase.
[0034] 3) Wash the organic phase until neutral. The spec...
Embodiment 1
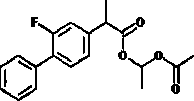
[0053] Embodiment 1: the preparation of flurbiprofen axetil.
[0054] Put 1.23mol of flurbiprofen and 0.15mol of p-toluenesulfonic acid in a reactor containing 6.0L of acetonitrile, heat up to 65°C, then slowly add 2.25mol of 1,1-ethylene glycol diacetic acid dropwise Esters, constant temperature reaction to the end, to obtain a reaction system.
[0055] The reaction system was concentrated by rotary evaporation under reduced pressure to remove the solvent. The heating temperature of rotary evaporation under reduced pressure was 50°C and the vacuum degree was -0.1mPa, and then 3.0L of Ethyl acetate and 3.0L of water were dissolved, and the organic phase was obtained by standing and separating.
[0056] Wash 3 times with 3.0L of saturated sodium bicarbonate solution, then wash 2 times with 3.0L of water until neutral, dry with 30g of anhydrous sodium sulfate for 8h, then carry out rotary evaporation and concentration under reduced pressure to remove the solvent, and decompress...
Embodiment 2
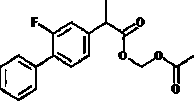
[0062] Embodiment 2: the preparation of flurbiprofen methylene diacetate.
[0063] Put 0.13mol of flurbiprofen and 0.52mol of acid catalyst in a reactor containing 0.6L of acetonitrile, heat up to 88°C, then slowly add 0.49mol of methylene diacetate dropwise, and keep the temperature until the end of the reaction , to get the reaction system.
[0064] The reaction system was concentrated by rotary evaporation under reduced pressure to remove the solvent. The heating temperature of rotary evaporation under reduced pressure was 65°C and the vacuum degree was -0.086mPa, and then 0.3L of Ethyl acetate and 0.3 L of water were dissolved, and the organic phase was obtained by standing and separating.
[0065] Wash 3 times with 0.3L saturated sodium bicarbonate solution, then wash 2 times with 0.3L water until neutral, use 15g of calcium sulfate to dry for 5h, then carry out vacuum rotary evaporation and concentration treatment to remove solvent, and vacuum rotary evaporation The he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com