Method for detecting related substances in flurbiprofen axetil drug
A technology of flurbiprofen axetil and a detection method, applied in the field of medicine, can solve the problems such as peak separation not meeting requirements, baseline drift and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Chromatographic conditions
[0032] Chromatograph model: Agilent 1260,
[0033] The column is Kinetex F5 (4.6×250mm, 5μm),
[0034] Mobile phase: acetonitrile: water-40:60 (volume ratio) isocratic elution,
[0035] Detection wavelength: 254nm,
[0036] Column temperature: 50°C,
[0037] Flow rate: 1ml / min.
[0038] Preparation of the test solution
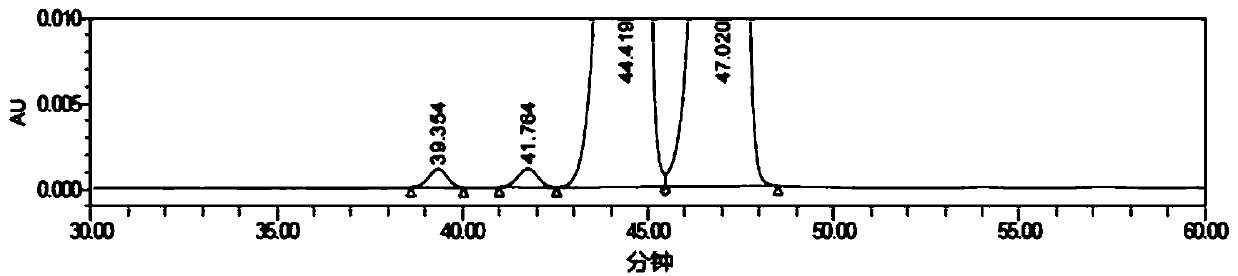
[0039] Get flurbiprofen axetil injection 1ml (equivalent to flurbiprofen axetil 10mg), be placed in 20ml volumetric flask, add mobile phase and dilute to scale, shake up, as need testing solution. According to the above chromatographic conditions, measure 20 μL and inject it into the liquid chromatograph, record the chromatogram, see the chromatogram figure 1 , the results are shown in the table below:
[0040]
[0041]
[0042] Under this chromatographic condition, the baseline is smooth. No. 1 peak (retention time 39.354min) and No. 2 peak (retention time 41.764min) are two pairs of enantiomers of deflurbip...
Embodiment 2
[0044] Chromatographic conditions
[0045] Chromatograph model: Waters e2695,
[0046] The column is Kinetex F5 (4.6×250mm, 5μm),
[0047] Mobile phase: acetonitrile: water-42:58 (volume ratio) isocratic elution,
[0048] Detection wavelength: 254nm,
[0049] Column temperature: 45°C,
[0050] Flow rate: 1.0ml / min.
[0051] Preparation of the test solution
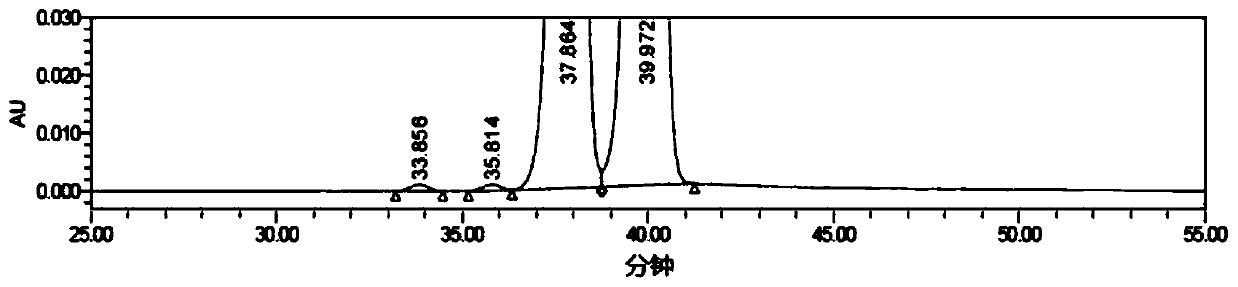
[0052] Get flurbiprofen axetil injection 1ml (equivalent to flurbiprofen axetil 10mg), be placed in 20ml volumetric flask, add dehydrated alcohol and dilute to scale, shake up, as need testing solution. According to the above chromatographic conditions, measure 20 μL and inject it into the liquid chromatograph, record the chromatogram, see the chromatogram figure 2 , the results are shown in the table below:
[0053] Peak ID retention time (min) %area Separation 1 33.856 0.130 21.92 2 35.814 0.119 2.07 3 37.864 45.658 2.00 4 39.972 53.942 1.83
[0054]Under this ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com