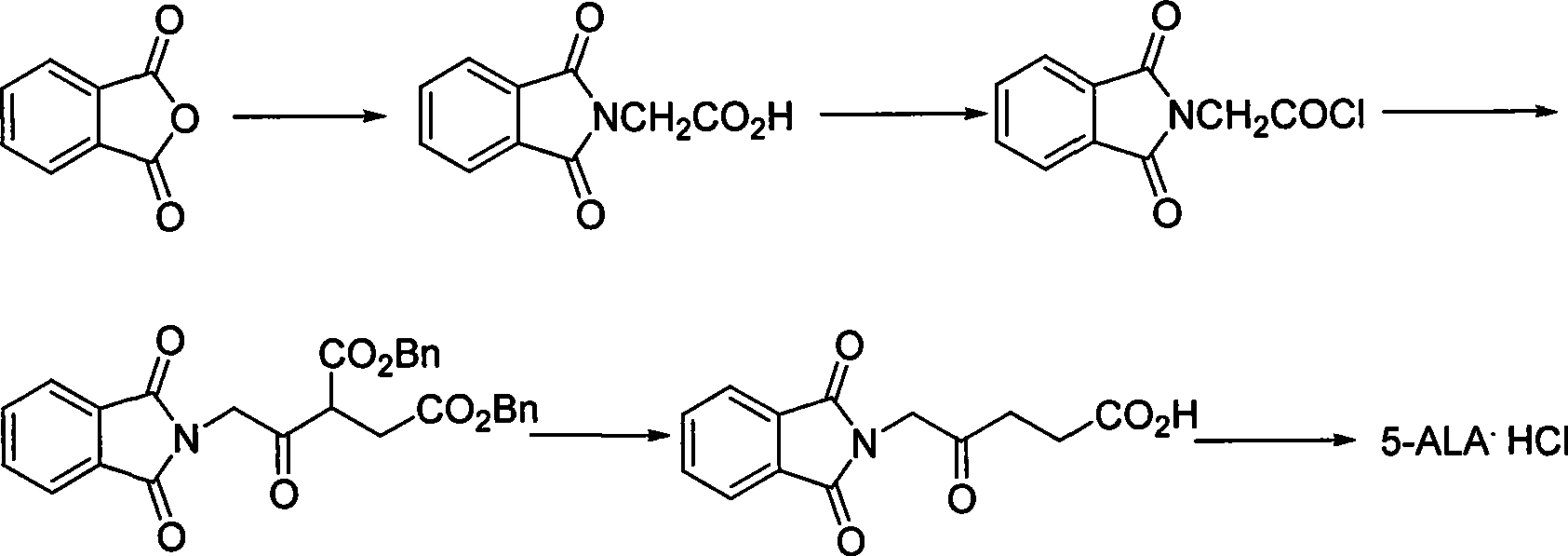
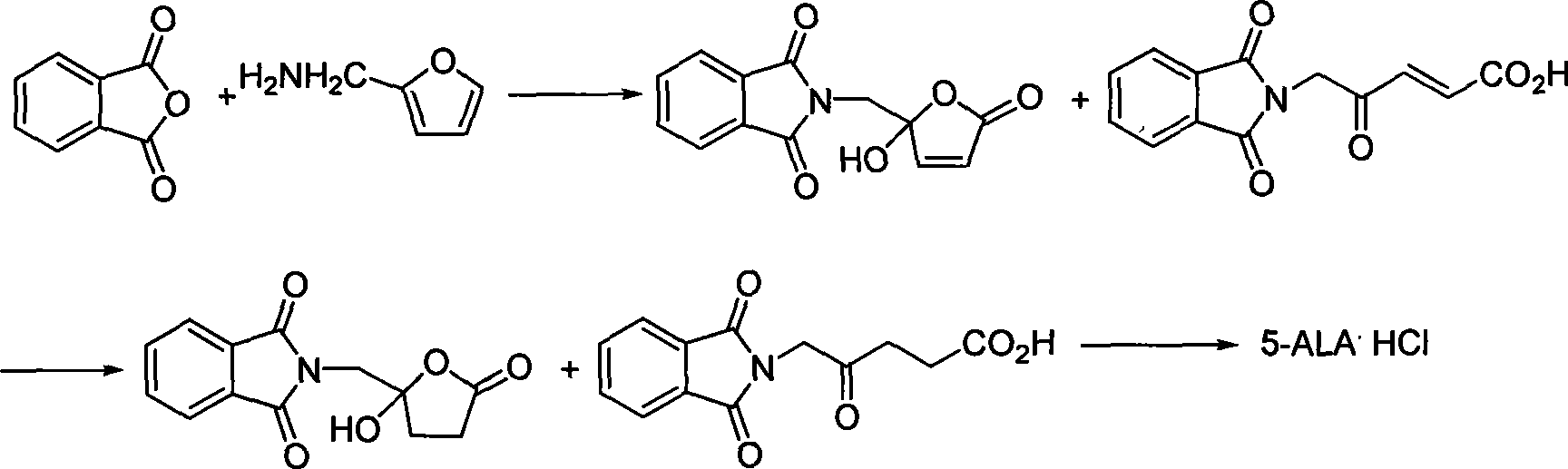
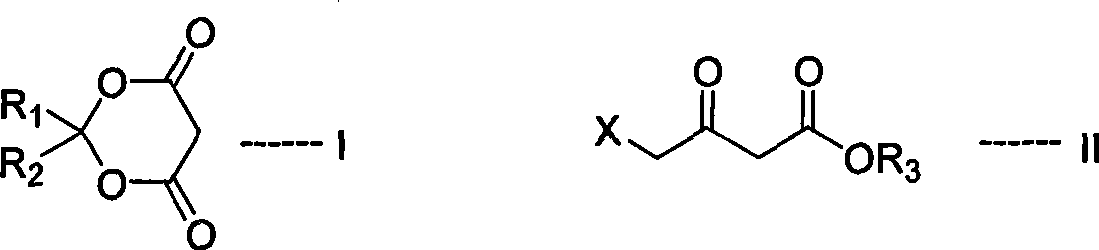Process for synthesizing 5-aminovaleric acid hydrochloride
A technology of amino ketovalerate hydrochloride and synthesis method, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve the problems of low production cost, low yield, high price and the like, and achieves easy operation. , the raw materials are easily available, and the effect is conducive to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]
[0048] In a 500ml four-necked flask, add 300ml of methanol, 15g of sodium methoxide and 25g of methylene malonate. After stirring at room temperature for 15 minutes, add 45g of ethyl bromoacetoacetate dropwise. After concentration, the resulting solid was washed with water, dried in vacuo and used directly in the next step.
[0049] Dissolve the product in the previous step in 300ml of absolute ethanol, add 5g of p-toluenesulfonic acid, heat to reflux, concentrate after the TLC detection reaction, and the obtained product is directly used in the next step.
[0050] Dissolve the product in the previous step in 250ml of acetic acid, cool to 0°C, add dropwise a solution of 15g of sodium nitrite dissolved in 50ml of water, control the dropping speed so that the temperature does not exceed 5°C, after adding, add 100ml of water, and slowly heat up After the reaction, a large amount of solid was precipitated, filtered by suction, dried and used in the next step.
[0051]...
Embodiment 2
[0055]
[0056] In a 500ml four-necked flask, add 300ml of dimethyl sulfoxide, 15g of potassium carbonate and 25g of methylene malonate, stir at room temperature for 15 minutes, add 45g of ethyl bromoacetoacetate dropwise, and heat to 100 degrees to react After the reaction was detected by TLC, the reaction solution was poured into ice water, and a large amount of white solid was precipitated, which was directly used in the next step after vacuum drying.
[0057] Dissolve the product in the previous step in 300ml of anhydrous methanol, add 5g of p-toluenesulfonic acid, heat to reflux, concentrate after the TLC detection reaction, and the obtained product is directly used in the next step.
[0058] Dissolve the product in the previous step in 250ml of acetic acid, cool to 0°C, add dropwise a solution of 15g of sodium nitrite dissolved in 50ml of water, control the dropping speed so that the temperature does not exceed 5°C, after adding, add 100ml of water, and slowly heat up A...
Embodiment 3
[0062]
[0063] In a 500ml four-necked bottle, add 300ml of dimethylformamide, 18g of sodium acetate and 25g of isopropylidene malonate, stir at room temperature for 15 minutes, add 40g of ethyl chloroacetoacetate dropwise, and heat to 100 After the reaction was detected by TLC, it was poured into ice water, and a large amount of solid was precipitated, filtered by suction, dried in vacuum and used directly in the next step.
[0064] Dissolve the product from the previous step in 300ml of anhydrous methanol, add 5g of HCl, heat to reflux, concentrate after the TLC detection reaction, and the obtained product is directly used in the next step.
[0065] Dissolve the product in the previous step in 250ml of acetic acid, cool to 0°C, add dropwise a solution of 15g of sodium nitrite dissolved in 50ml of water, control the dropping speed so that the temperature does not exceed 5°C, after adding, add 100ml of water, and slowly heat up After the reaction, a large amount of solid wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com