Preparation method of 1-methyl-3-pyrrolidinol
A technology of pyrrolidinol and methyl, which is applied in the field of preparation of 1-methyl-3-pyrrolidinol, can solve the problems of low yield and difficulty in large-scale production, so as to improve the purity, improve product quality and reduce the difficulty of purification Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
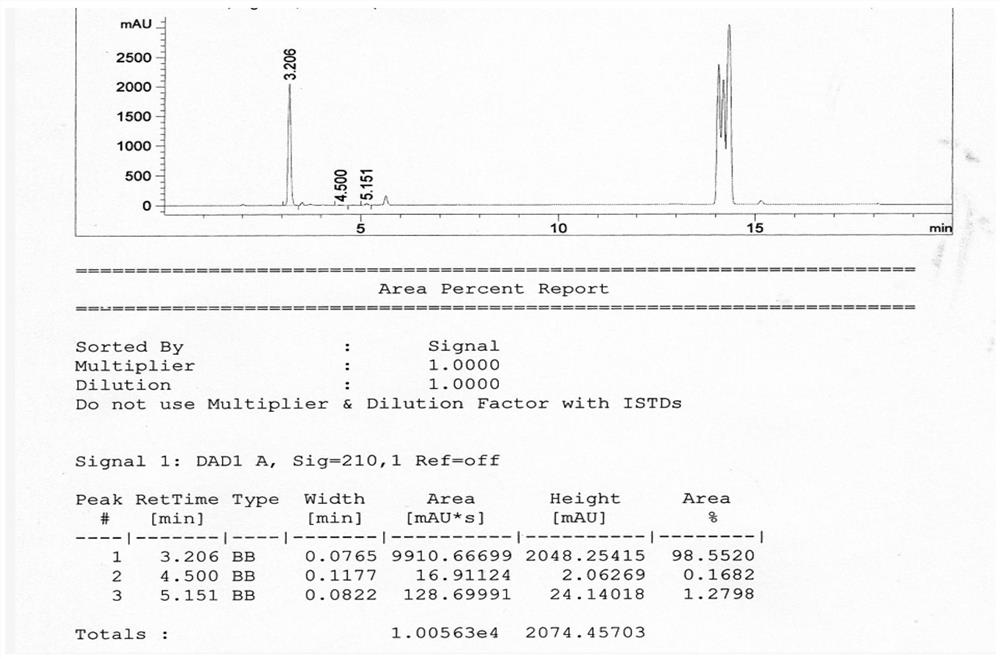
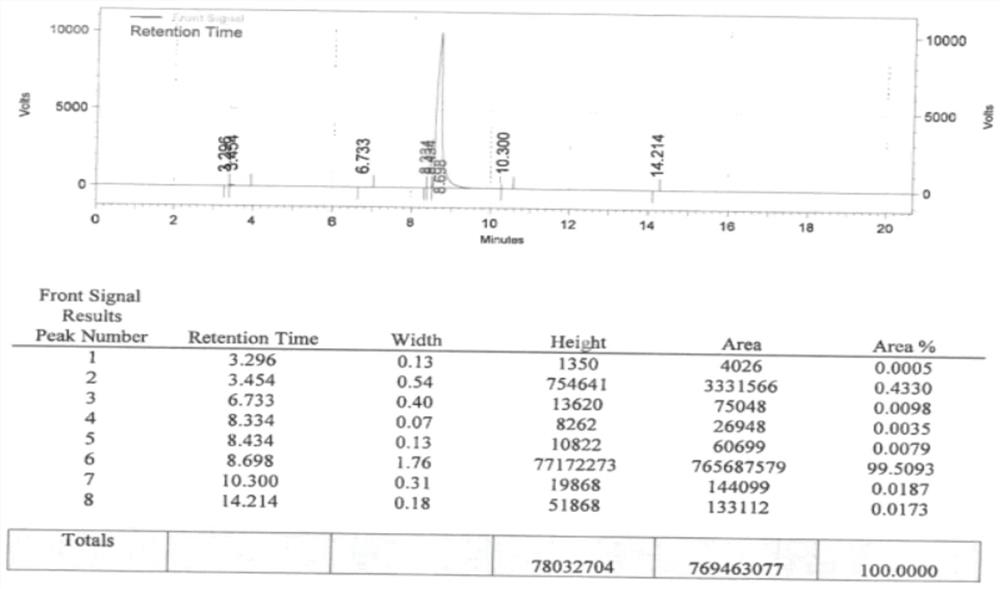
Image
Examples
Embodiment 1
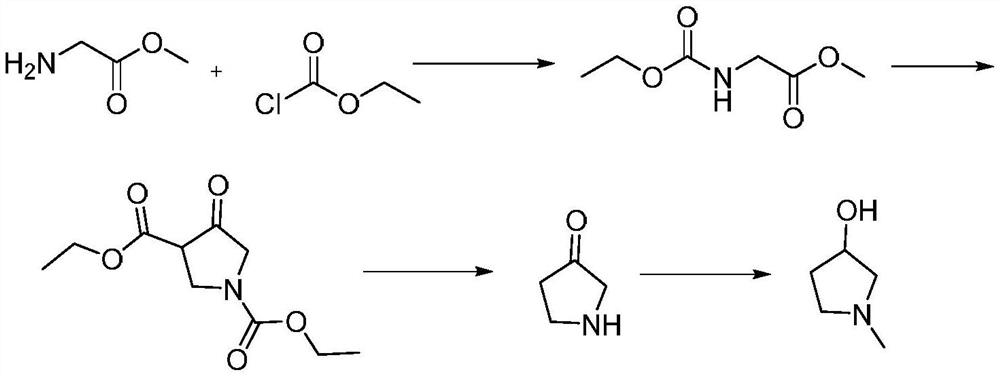
[0052] A preparation method of 1-methyl-3-pyrrolidinol, comprising the steps of:
[0053] S1. Compound I and Compound II are subjected to a ring-closing reaction to obtain Compound III;
[0054] S2, reducing the compound III obtained in step S1 and the reducing agent IV to obtain 1-methyl-3-pyrrolidinol;
[0055] Among them, compound I is Compound II is H 3 CNH 2 , compound III is
[0056] Step S1 specifically includes the following sub-steps:
[0057] S1-1. Take 420.0g of toluene (solvent A) and add it to a 1L reaction flask, take 60.0g of malic acid (compound I), and 41.6g of 40% aqueous solution of methylamine (compound II) and drop them into a 1L reaction flask, Control the temperature in the 1L reaction bottle to stir at 15°C, control the stirring time to 0.5h, heat to reflux in the 1L reaction bottle, and divide the water for 18 hours;
[0058]S1-2. Sampling and testing. After the reaction is over, cool down and concentrate to remove toluene (solvent A), add 6g ...
Embodiment 2
[0065] A preparation method of 1-methyl-3-pyrrolidinol, comprising the steps of:
[0066] S1. Compound I and Compound II are subjected to a ring-closing reaction to obtain Compound III;
[0067] S2, reducing the compound III obtained in step S1 and the reducing agent IV to obtain 1-methyl-3-pyrrolidinol;
[0068] Among them, compound I is Compound II is H 3 CNH 2 , compound III is
[0069] Step S1 specifically includes the following sub-steps:
[0070] S1-1. Take 420.0g of xylene (solvent A) and add it to a 1L reaction flask, take 60.0g of malic acid (compound I), and 43.2g of 40% aqueous solution of methylamine (compound II) and add them dropwise to a 1L reaction flask In the process, the temperature in the 1L reaction bottle was controlled to be stirred at 15°C, the stirring time was controlled to be 0.5h, heated to reflux in the 1L reaction bottle, and the water was separated for 14 hours;
[0071] S1-2. Sampling and testing. After the reaction is over, cool down a...
Embodiment 3
[0078] A preparation method of 1-methyl-3-pyrrolidinol, comprising the steps of:
[0079] S1. Compound I and Compound II are subjected to a ring-closing reaction to obtain Compound III;
[0080] S2, reducing the compound III obtained in step S1 and the reducing agent IV to obtain 1-methyl-3-pyrrolidinol;
[0081] Among them, compound I is Compound II is H 3 CNH 2 , compound III is
[0082] Step S1 specifically includes the following sub-steps:
[0083] S1-1. Take 420.0g of chlorobenzene (solvent A) and add it to a 1L reaction flask, take 60.0g of malic acid (compound I), and 43.2g of 40% aqueous solution of methylamine (compound II) and drop them into a 1L reaction flask , control the temperature in the 1L reaction bottle to stir under the condition of 15°C, control the stirring time to 0.5h, heat to reflux in the 1L reaction bottle, and divide the water for 10 hours;
[0084] S1-2. Sampling and testing. After the reaction is finished, reduce the temperature and conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com