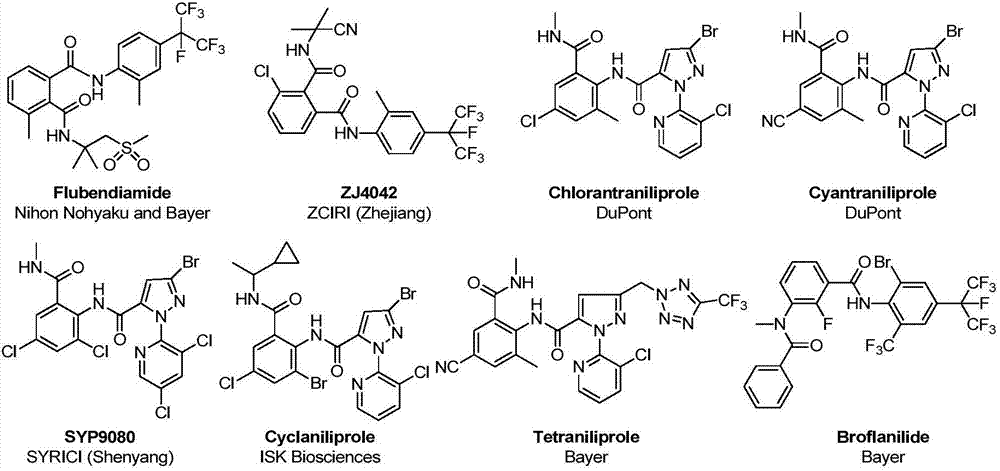
Amide derivative containing alpha-aminoketone structure and preparation method and application thereof
The technology of amide derivatives and amino ketones is applied in the field of amide derivatives containing an α-amino ketone structure and the preparation thereof, which can solve the problems of outstanding pest resistance and other problems, and achieve the effects of simple and easy preparation process and easy availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
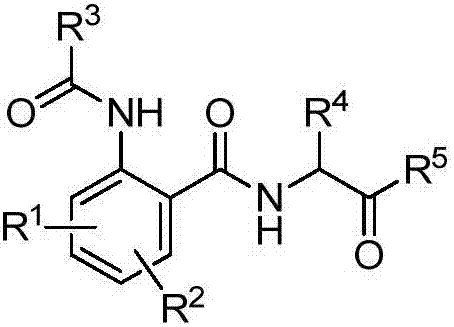
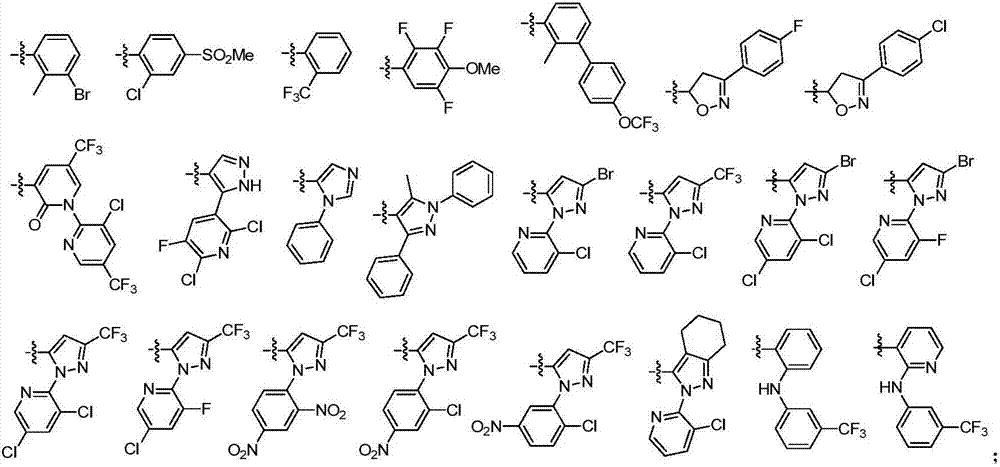
[0033] The structural formula of some amide derivatives containing α-aminoketone structure of the present invention is one of the specific compounds listed in Table 1:
[0034] Table 1: Representative compounds shown in structural formula I
[0035]
[0036]
[0037]
[0038]
[0039]
[0040]
[0041]
[0042]
[0043]
[0044]
Embodiment 2
[0046] Numbering is the preparation of the compound of I1 among the embodiment 1, and its reaction formula is:
[0047]
[0048] Its preparation method comprises the following steps:
[0049] a) Using anthranilic acid (compound 1a) and 3-bromo-2-methylbenzoic acid (compound 2a) as starting materials, the heterocyclization reaction was carried out under the combined action of methanesulfonyl chloride and pyridine to obtain intermediate 2 -(3-bromo-2-methylphenyl)-4H-benzoxazin-4-one (compound 3a), the reaction temperature is 20°C, and the reaction solvent is 1,2-dichloroethane;
[0050]b) Using the intermediate 2-(3-bromo-2-methylphenyl)-4H-benzoxazin-4-one (compound 3a) as the starting material, it undergoes a substitution reaction with isopropanolamine 4a to obtain Ring-opening intermediate 5a, the reaction temperature is 30°C, and the reaction solvent is acetonitrile;
[0051] c) The intermediate 5a was oxidized under the action of oxidant m-chloroperoxybenzoic acid (MC...
Embodiment 3
[0053] Numbering is the preparation of the compound of I9 among the embodiment 1, and its reaction formula is:
[0054]
[0055] Its preparation method comprises the following steps:
[0056] a) Starting with 2-amino-5-chloro-3-methylbenzoic acid (compound 1b) and 2-chloro-4-thiamphenicol benzoic acid (compound 2b), in the common combination of methanesulfonyl chloride and pyridine The heterocyclization reaction under the influence of The reaction temperature is 25°C, and the reaction solvent is tetrahydrofuran;
[0057] b) starting from the intermediate 6-chloro-2-(2-chloro-4-thiamphenicolphenyl)-8-methyl-4H-benzoxazin-4-one (compound 3b), and Isopropanolamine 4a is subjected to substitution reaction to obtain ring-opening intermediate 5b, the reaction temperature is 30°C, and the reaction solvent is dichloromethane;
[0058] c) Dissolving the intermediate 5b in an appropriate amount of tetrahydrofuran, and performing an oxidation reaction using Sarrett reagent (PCC) to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com