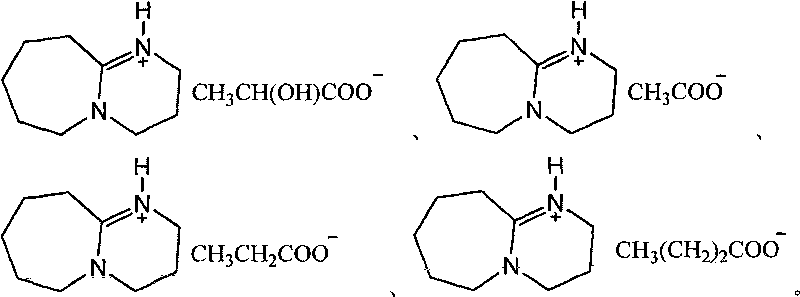
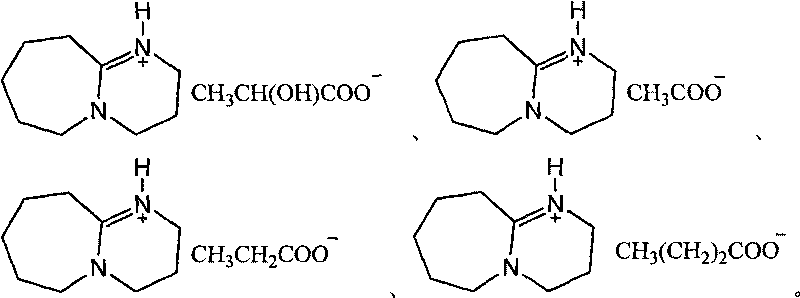
Novel method for preparing beta-aminoketone, ester, nitrile and amide derivatives through catalysis of functional ionic liquid
An ionic liquid, nitrile and amide technology, applied in chemical instruments and methods, organic chemistry methods, cyanide reaction preparation, etc., can solve the problems of excess, harsh reaction conditions, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
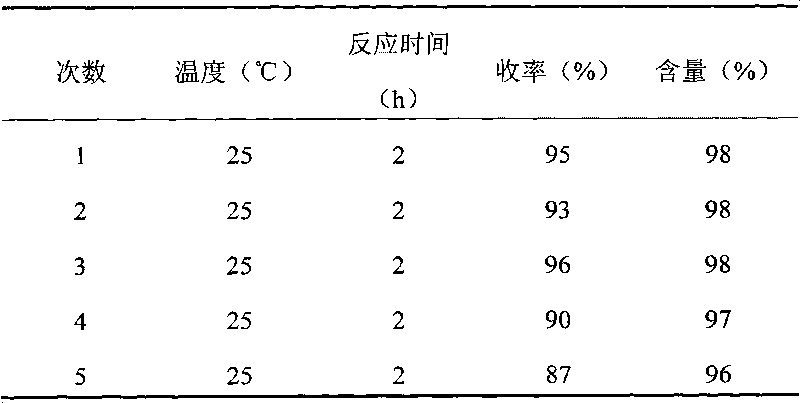
[0023] Morpholine (5mmol), methyl acrylate (5.5mmol), and 1mmol lactic acid ionic liquid [DBU][Lac] were sequentially added to a 50mL single-necked bottle, stirred at room temperature for 2 hours, and detected by TLC, the raw materials disappeared, and the reaction solution was extracted with ether. The organic phases were combined and separated by column chromatography to obtain the product with a yield of 95% and a content of 98%. 3-(1-Morpholinyl)-propionic acid methylester: 1 H NMR (400MHz, CDCl 3 )(ppm): 3.66(s, 3H, OCH 3 ), 3.67-3.65 (m, 4H, morpholinyl), 2.65 (t, 2H, J=6Hz, CH 2 ), 2.48(t, 2H, J=6Hz, CH 2 ), 2.43-2.41 (m, 4H, morpholinyl); 13 C NMR (100MHz, CDCl 3 )(ppm): 172.8, 66.8, 53.8, 53.3, 51.6, 31.7.
Embodiment 2
[0025] Add n-propylamine (5mmol), methyl acrylate (5.5mmol), and 1mmol n-propionic acid ionic liquid [DBU][Pr] into a 50mL single-necked bottle in turn, stir at room temperature for 2 hours, TLC detects that the raw materials disappear, and extract the reaction with ether liquid, combined the organic phases, and separated by column chromatography to obtain the product with a yield of 86% and a content of 95%. 3-(1-Propylamine)-propionic acidmethyl ester: 1 H NMR (400MHz, CDCl 3 )(ppm): 3.63(s, 3H, OCH 3 ), 2.62(t, 2H, J=6.4Hz, CH 2 ), 2.45(t, 2H, J=6.4Hz, CH 2 ), 2.58 (m, 2H, CH 2 ), 1.41 (m, 2H, CH 2 ), 0.90 (m, 3H, CH 3 ); 13 C NMR (100MHz, CDCl 3 )(ppm): 170.5, 66.8, 52.8, 52.1, 44.9, 28.1, 12.3.
Embodiment 3
[0027] Piperidine (5mmol), methyl acrylate (5.5mmol), and 1mmol acetic acid ionic liquid [DBU][Ac] were sequentially added to a 50mL single-necked bottle, stirred at room temperature for 1.5 hours, and detected by TLC, the raw materials disappeared, and the reaction solution was extracted with ether. The organic phases were combined and separated by column chromatography to obtain the product with a yield of 96% and a content of 98%. 3-(1-Piperidinyl)-propionic acidmethyl ester: 1 H NMR (400MHz, CDCl 3 )(ppm): 3.86(s, 3H, OCH 3 ), 2.86(t, 2H, J=6Hz, CH 2 ), 2.68(t, 2H, J=6Hz, CH 2 ), 1.80-1.75 (m, 4H, piperidinyl), 1.61-1.44 (m, 4H, piperidiny), 1.42-1.26 (m, 2H, piperidinyl); 13 C NMR (100MHz, CDCl 3 )(ppm): 173.8, 54.2, 53.8, 52.9, 31.8, 29.3, 25.8, 24.6, 23.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com