Escherichia coli recombinant bacteria capable of high-producing 2, 5-dimethylpyrazine and construction method of escherichia coli recombinant bacteria
A technology of dimethylpyrazine and Escherichia coli, applied in the field of genetic engineering, can solve the problems of influence, imbalance of supply and consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
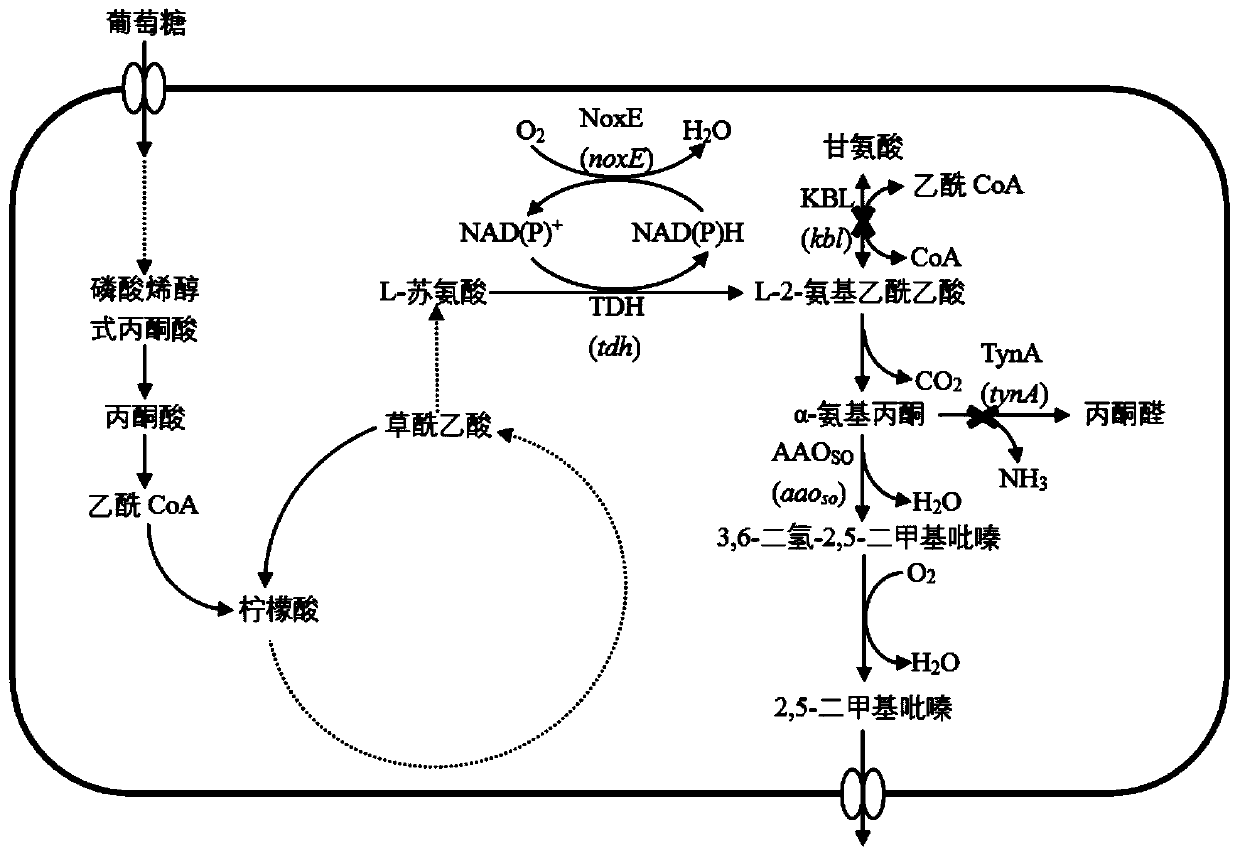
[0042] Example 1: Recombinant plasmid pEC-XK99E-tdh-noxE-aao so build
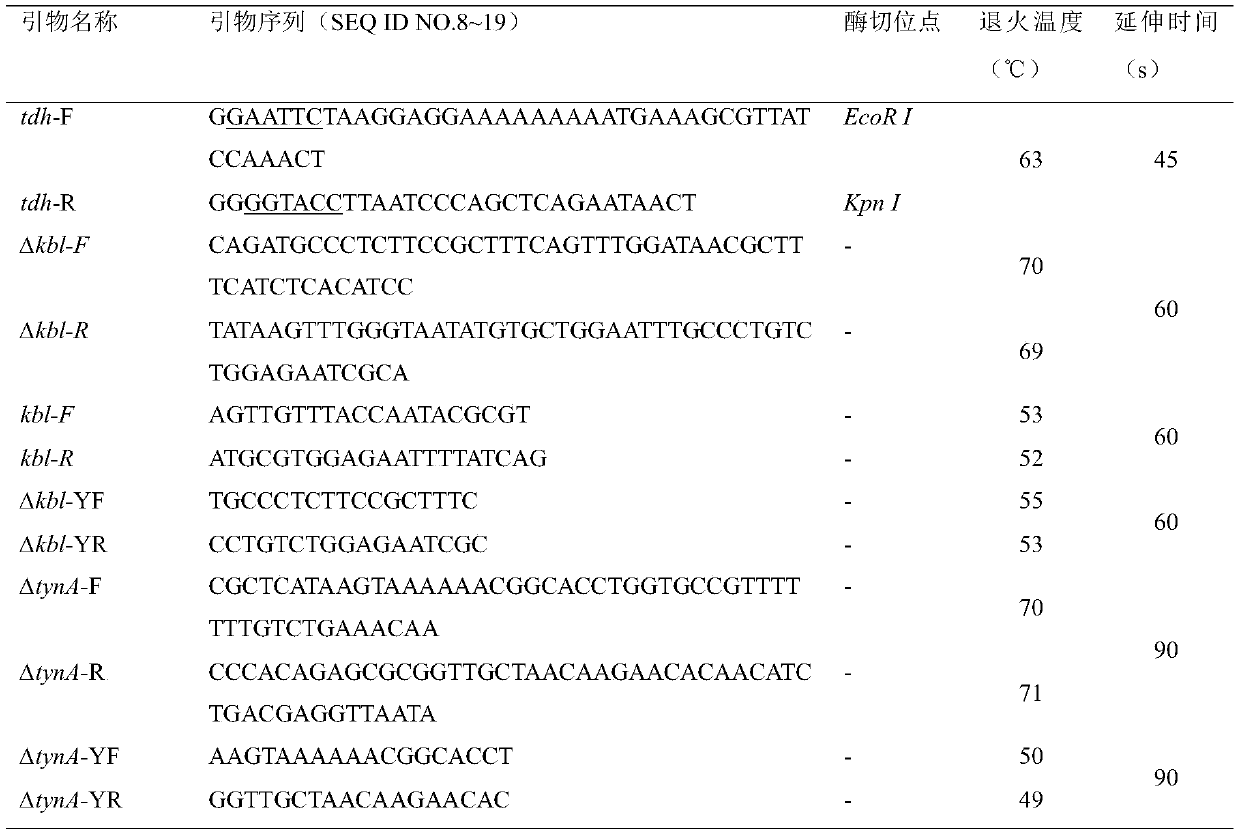
[0043] According to the E.coli K-12tdh gene sequence in GenBank (GenBank accession number U00096REGION: 3790320..3791345). Restriction endonuclease EcoR I and Kpn I restriction site sequences were added to the upstream and downstream of the gene, and the Escherichia coli SD recognition sequence TAAGGAGGAAAAAAAAA was added upstream to design primers tdh-F and tdh-R. Use primers tdh-F and tdh-R to carry out PCR with the Escherichia coli K-12 genome as a template, and gel recovery to obtain the target gene fragment. Subsequently, the plasmid pEC-XK99E and the tdh fragment of the target gene were digested with restriction enzymes EcoR I and Kpn I, and the linear pEC-XK99E and tdh fragments were purified using a product purification kit. The recovered linear pEC-XK99E and tdh fragments were treated with T 4 After DNA ligase enzyme ligation, transform into JM109, colony PCR picks positive transformants, and e...
Embodiment 2
[0046] Example 2: Knockout of the starting bacterium E.coli THR 2-amino-3-ketobutyrate CoA ligase gene kbl and primary amine oxidase gene tynA
[0047] The first step is to replace the target gene to be knocked out with the kan gene: use pKD13 as a PCR template, and amplify the primers Δkbl-F and Δkbl-R to obtain a DNA fragment (including the upstream and downstream homology arm sequences of kbl, the kan gene and two FRT site) was transformed into E.coli / pKD46 competent cells with a 1mm electric shock cup at a voltage of 1800V. After electric shock, the cells were re-incubated at 37°C for 2h and spread on LBK25 plates. Under the induction of arabinose, pKD46 expresses the recombinant protein, and mediates the recombination between the homology arm gene of the knockout frame and the E. coli genome. After culturing for 12 hours, a single colony was picked, and PCR amplification was performed using primers kbl-F and kbl-R, which were designed inside the kbl gene. If no kbl gene ...
Embodiment 3
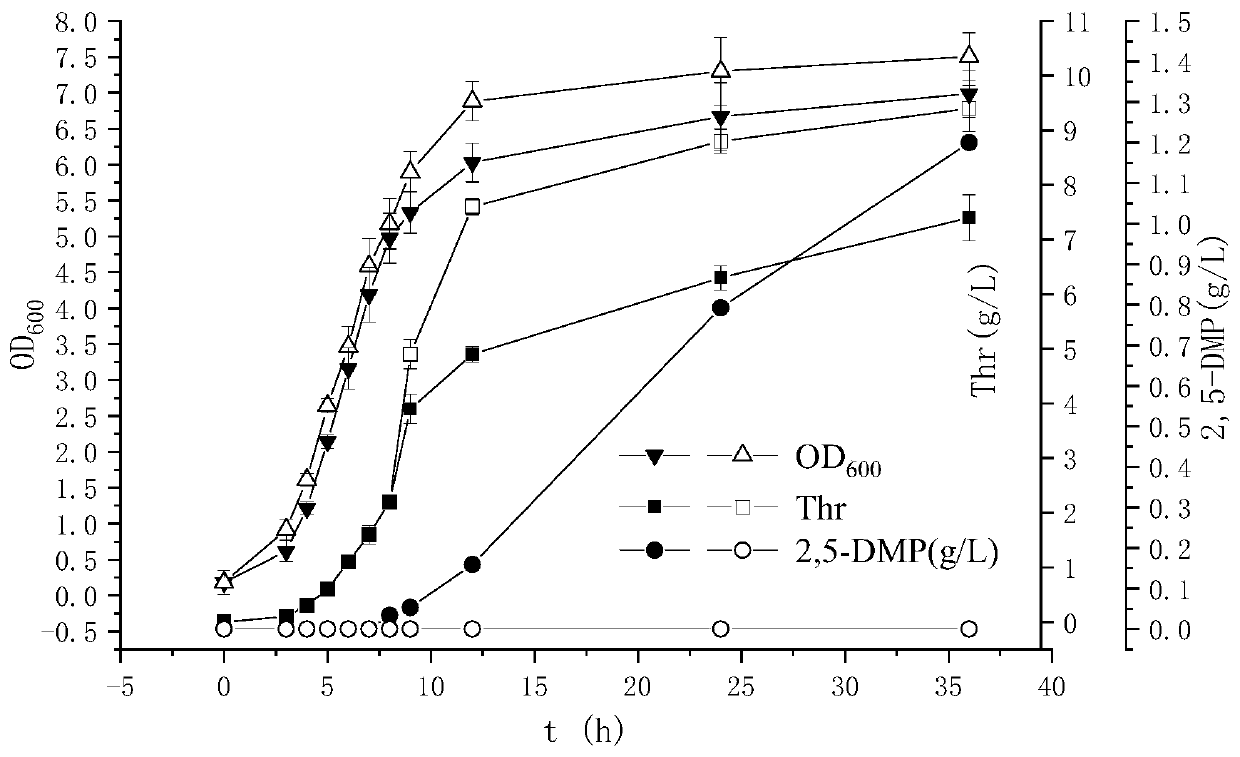
[0050] Embodiment 3: Threonine dehydrogenase TDH, NADH oxidase NoxE, aminoacetone oxidase AAO in recombinant bacteria and starting strain SO Enzyme activity assay
[0051] Determination of TDH: Inoculate the strains stored in the frozen tube into LB liquid medium containing kanamycin (50 μg / ml), culture with shaking at 37°C and 100 rpm for 10 hours, and scale up the culture to 100ml containing kanamycin according to 1% inoculum size. Mycin (50 μg / ml) in an LB Erlenmeyer flask (500 ml), induced at 37° C. and 100 rpm for 16 h, and centrifuged at 4° C. and 8000 rpm to collect the bacteria. The collected bacteria were washed twice with 0.85% saline. Subsequently, the cells were suspended in Tris-HCl buffer (20 mmol / L, pH 8.0) containing 150 mmol / L NaCl and ultrasonically disrupted to prepare a crude enzyme solution. Determination of TDH enzyme activity: NADH has a maximum absorbance value at 340nm, and TDH activity is calculated by detecting the change of NADH absorbance value a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com