Plasmid for efficiently catalyzing L-threonine to synthesize 2, 5-DMP and construction and application of plasmid
A technology of threonine and threonine dehydrogenase, which is applied in the direction of nucleic acid carriers, fusion polypeptides, oxidoreductases, etc., can solve the problems of difficult product separation, low overall conversion rate, long reaction pathway, etc., and achieve the improvement of cofactor unbalanced effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Cloning of threonine dehydrogenase gene tdh and construction of recombinant bacteria
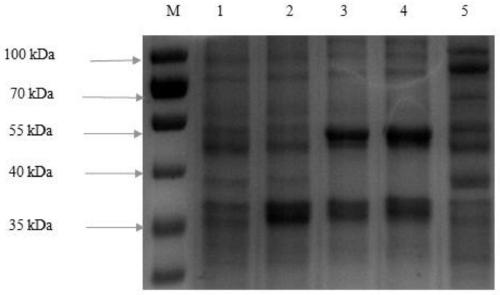
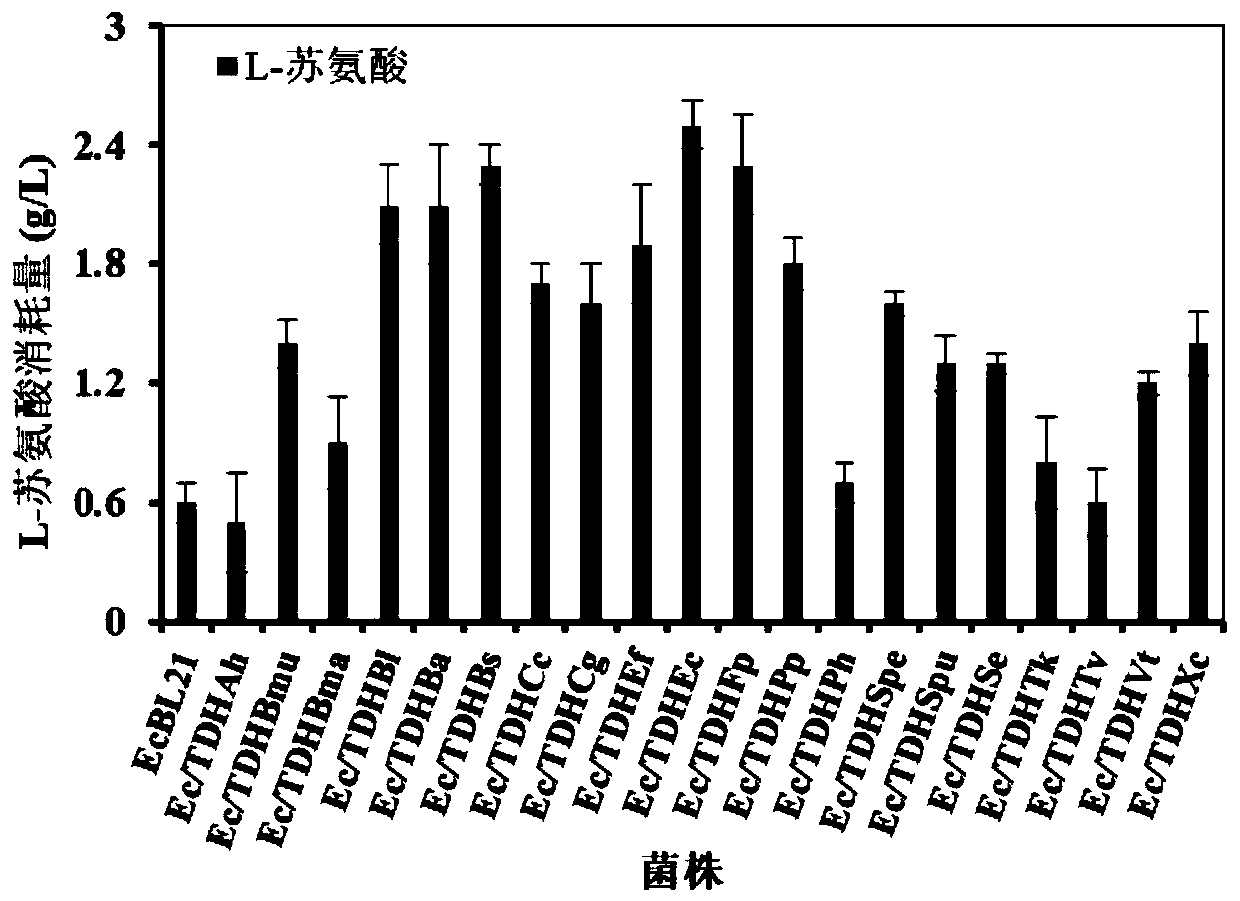
[0040] Based on the National Center for Biotechnology Information (NCBI) database, a threonine dehydrogenase (TDH) phylogenetic tree was constructed, and 20 TDHs from representative microorganisms were selected ( figure 2 ). The TDH amino acid sequences of 20 sources were obtained from the GeneBank database, except for the tdh gene derived from Escherichia coli K-12 through the primer tdh Ec -F / R was obtained by PCR using the Escherichia coli K-12 genome as a template, and the rest were submitted to Suzhou Jinweizhi Biotechnology Co., Ltd. to optimize, synthesize and connect to the expression vector pRSFDuet-1 according to the codon preference of Escherichia coli. Between the sites BamHI and EcoRI, obtain the recombinant plasmid pRSFDuet-tdh X ( X represent different microbial sources). The recombinant plasmid pRSFDuet-tdh X Transform into competent cells E.coli BL...
Embodiment 2
[0041] Example 2: Determination of enzyme activity of threonine dehydrogenase TDH, selecting the TDH with the highest activity for subsequent construction
[0042] Determination of TDH: Inoculate the strains stored in the frozen tube into LB liquid medium containing kanamycin (50 μg / ml), culture with shaking at 37°C and 100 rpm for 10 hours, and scale up the culture to 100ml containing kanamycin according to 1% inoculum size. Mycin (50 μg / ml) in an LB Erlenmeyer flask (500 ml), induced at 37° C. and 100 rpm for 16 h, and centrifuged at 4° C. and 8000 rpm to collect the bacteria. The collected bacteria were washed twice with 0.85% saline. Subsequently, the cells were suspended in Tris-HCl buffer (20 mmol / L, pH 8.0) containing 150 mmol / L NaCl and ultrasonically disrupted to prepare a crude enzyme solution. Determination of TDH enzyme activity: NADH has a maximum absorbance value at 340nm, and TDH activity is calculated by detecting the change of NADH absorbance value at 340nm d...
Embodiment 3
[0046] Example 3: Cloning of NADH oxidase gene noxE and construction of recombinant bacteria
[0047] Obtain the NADH oxidase (NoxE) gene sequence (Accession No.AM406671.1) in Lactococcus cremoris MG1363 from the GeneBank database, and submit it to Suzhou Jinweizhi Biotechnology Co., Ltd. for optimization according to the codon preference of Escherichia coli (nucleic acid before optimization The sequence is shown in SEQ ID NO.6, and the optimized nucleic acid sequence is shown in SEQ ID NO.7), synthesized and connected to the expression vector pRSFDuet-tdh Ec Between EcoRI and HindIII of the enzyme cutting site, the recombinant plasmid pRSFDuet-tdh was obtained Ec -noxE Lc , or connected to the expression vector pRSFDuet-tdh Ec Between NdeI and XhoI of the enzyme cutting site, the recombinant plasmid pRSFDuet-tdh was obtained Ec -PnoxE Lc . In addition, the fusion protein was designed according to the amino acid sequence of TDH and NoxE derived from Escherichia coli K-12,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com