Solid compositions comprising 5-aminolevulinic acid
A composition and solid-state technology, which can be used in medical preparations containing active ingredients, drug combinations, and medical preparations with non-active ingredients, etc., and can solve problems such as delayed culture period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Embodiment 1: solid composition among the present invention
[0181] Coated capsules containing n-hexylaminolevulinate (HAL) hydrochloride (HAL HCl) (Phototherapy ASA, Norway) by mixing the compounds listed under "Capsule Composition" at a temperature above their melting point to prepare. The mixture was poured into HPMC capsules and banded with a mixture of HPMC (3.1 mg), gellan gum (0.015 mg) and trisodium citrate (0.05 mg) in water. The capsules are moisture-proof coating (6.3mg / cm 2 ) and Opadry (AMB) coating, followed by enteric coating (8mg / cm 2 , for 80% of the L 30D-55 and 20% FS 30D, both dispersed in water) to form a pH-sensitive film that decomposes at pH 6.5 and above.
[0182]
[0183] Of all the capsules containing Miglyol 812 as triglyceride, capsule A is a solid pharmaceutical product outside the scope of the present invention as it contains no emulsifier. Capsules B and C contain anionic emulsifiers, and capsules D to H contain nonionic emu...
Embodiment 2
[0184] Example 2: Dissolution of HAL
[0185] Capsules A-H prepared in Example 1 were used for in vitro dissolution studies. In order to simulate the human intraperitoneal environment, the capsule was first immersed in 500 ml of 0.1M HCl dissolution medium (1) at a temperature of 37° C. for one hour. The capsules were then taken out of the medium and immersed in 500 ml of pH 6.5 phosphate buffered aqueous solution (2) at a temperature of 37° C. to simulate the internal environment of the human terminal ileum, ie the water environment and pH. A "USP 711" complying dissolution apparatus equipped with paddles and sinkers was used in two immersions. Capsules are placed on the sinker and immersed in the dissolution medium. The rotation speed was set at 75 rpm. 2 ml samples of dissolution medium were manually withdrawn at 5, 15, 30, 60, 120 and 180 minutes, respectively. The sample was filtered (40 μm HDPE filter membrane) and its HAL content was detected by HPLC. The HAL con...
Embodiment 3
[0196] Example 3: In vivo release of HAL HCl
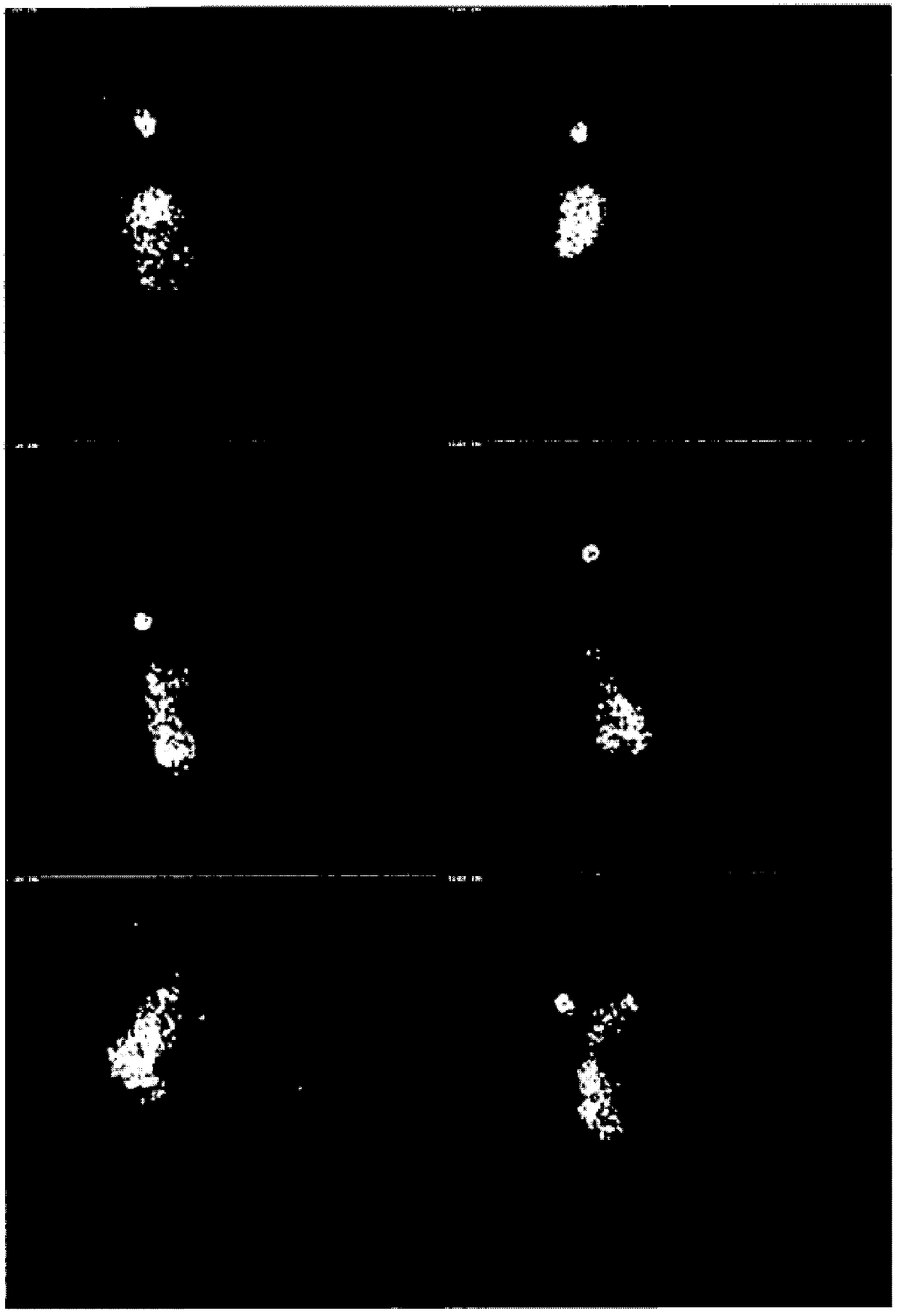
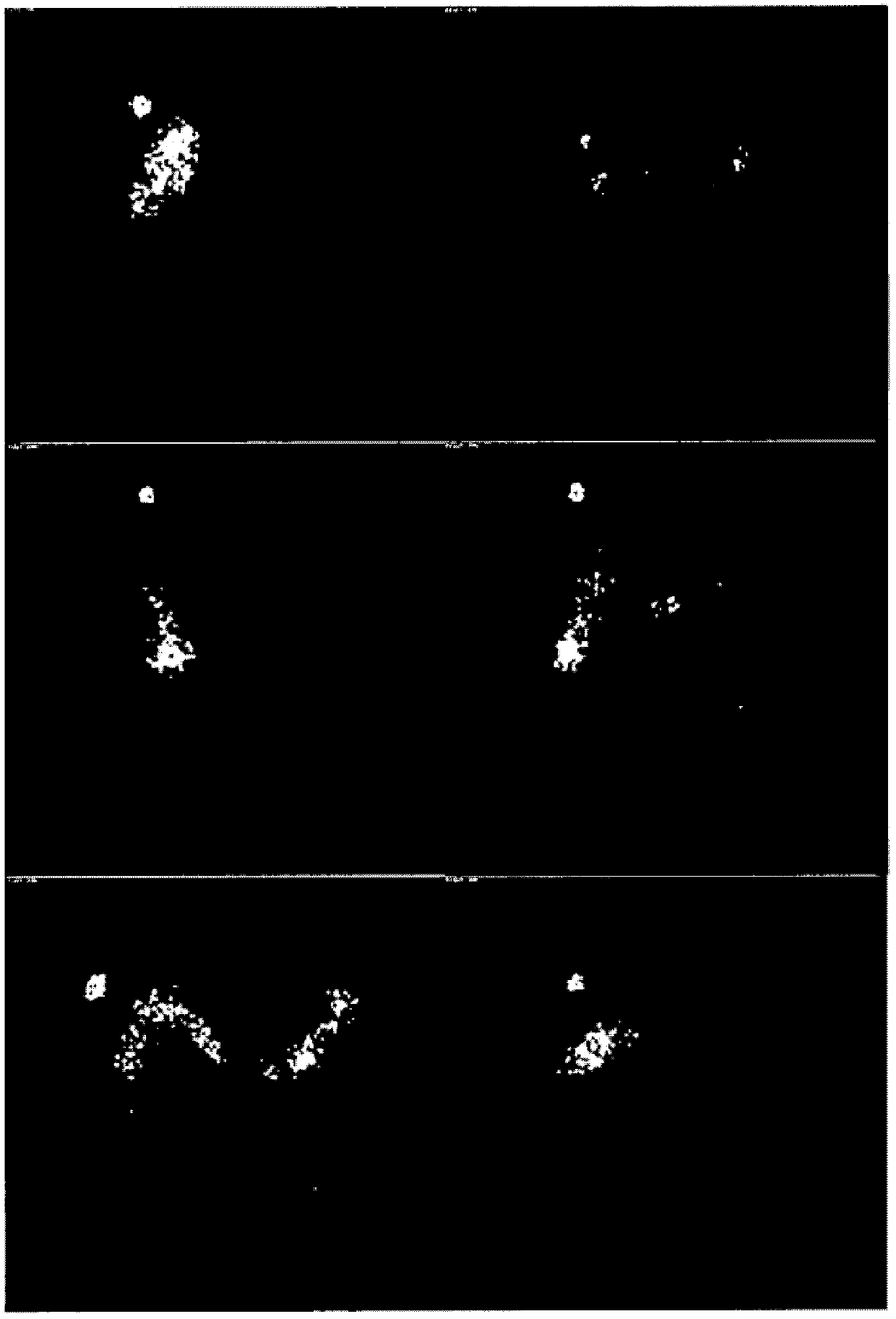
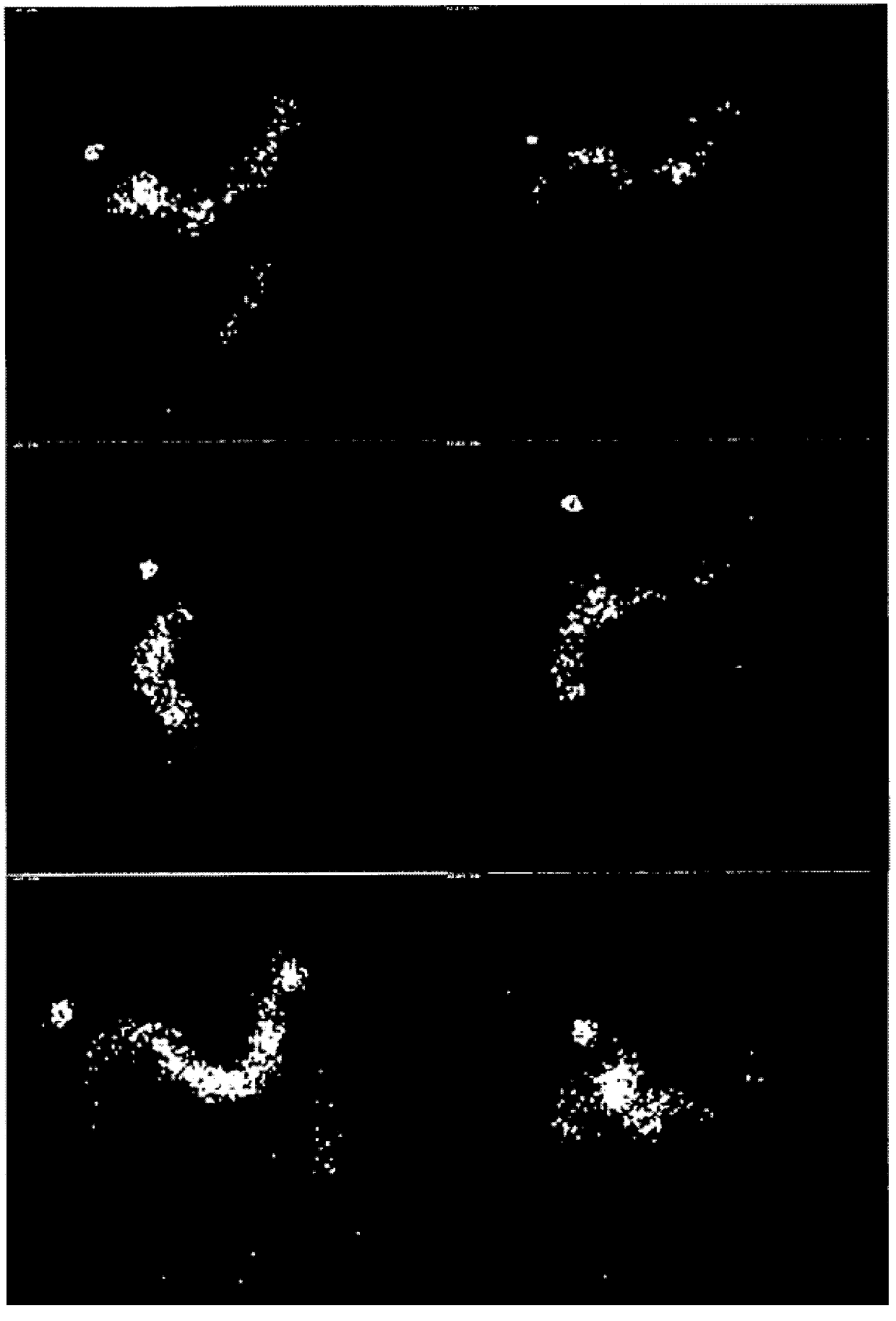
[0197] In order to evaluate the transfer of enteric-coated capsules, that is, the solid pharmaceutical product of the present invention, in the gastrointestinal tract to determine the release location of the composition in the enteric-coated capsules and the release of HAL HCl in the emptied, i.e., cleared colon. distribution, we performed gamma-scan imaging studies on healthy male volunteers.
[0198] Gamma scan imaging can assess the physical integrity of the solid pharmaceutical product of the present invention as it moves through the gastrointestinal tract. Detailed information on the timing and anatomical location of the breakdown of the drug can thus be obtained. will Enterion TM Site-specific delivery capsules are used in gamma-scan imaging studies, thus allowing drug delivery to specific sites within the GI tract. The capsule is 35mm long and 10-12mm in diameter, and can deliver solutions, suspensions or powders to sp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com