5-aminolevulinic acid (ALA) synthetase mutant and host cells and application thereof
A host cell, amino acid technology, applied to mutants of 5-aminolevulinic acid synthase and its host cells and application fields, can solve the problem of not many, no ALA biosynthesis, no research on the thermostability and resistance of mutant enzymes Hemoglobin feedback inhibition ability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Embodiment 1. Construction of ALA synthetase mutation carrier
[0146] Utilize the Stratagene Series Site-directed mutagenesis kit, design 2 pairs of primers (see Table 1), use pET21a-hemA (refer to Zhang et al. Biotechnology Letters, 2013, 35(5): 763-768) wild-type plasmid as a template, use the above primers Perform PCR amplification, respectively mutate the arginine (R) at the 40th and 365th positions of HemA to glycine (G) and lysine (K), the PCR reaction conditions are: 95 ° C for 5 min, 10 cycles (95 ℃30s, 74℃-65℃30s, 68℃7min), 13 cycles (95℃30s, 65℃30s, 68℃7min), 68℃10min. PCR amplification system (50 μL): template 1 μL, upstream and downstream primers 2 μL, dNTP mix 1 μL, 10×Pyrobest Buffer 5 μL, sterilized double distilled water 38.5 μL, Pyrobest DNA polymerase 0.5 μL. PCR products were purified and recovered using a gel recovery kit. The transformants were sequenced by Jinweizhi Company, and the correctly sequenced expression vectors were named pET21a-R40G...
Embodiment 2
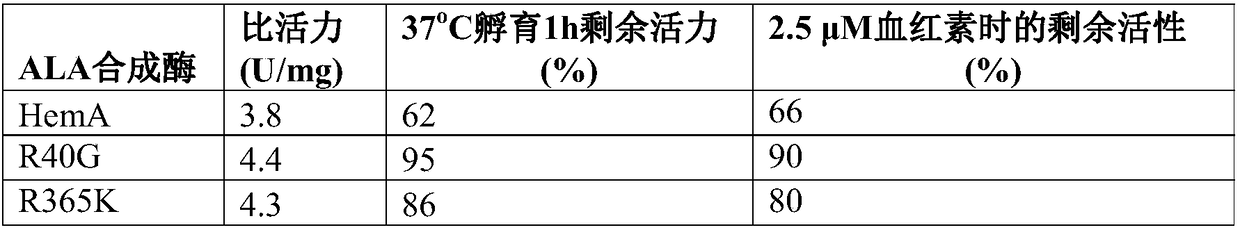
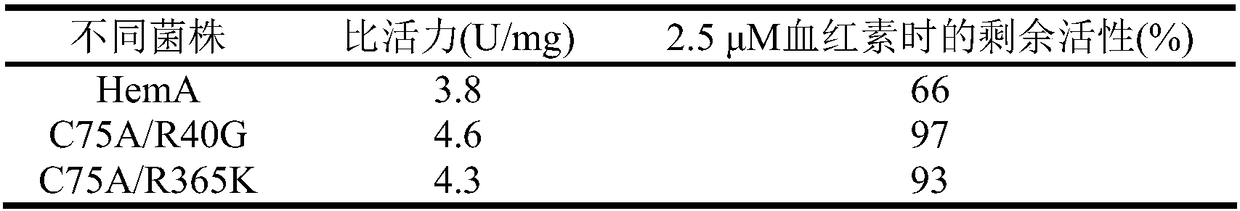
[0149] Example 2. Detection of ALA Synthetase Mutant Enzyme Enzymatic Properties
[0150] Transform the pET21a-hemA, pET21a-R40G and pET21a-R365K mutant plasmids into E.coliBL21(DE3), respectively, to obtain recombinant strains BL21(DE3) / pET21a-hemA, BL21(DE3) / pET21a-R40G, BL21(DE3) / pET21a-R365K, used for the expression of different enzymes and the detection of enzymatic properties.
[0151] Inoculate 5 mL of LB liquid medium containing 100 μg / mL ampicillin with a single colony of the above-mentioned recombinant bacteria, and culture at 37° C. and 220 rpm for 12 hours. Transfer to a 500mL Erlenmeyer flask filled with 100mL LB liquid medium containing 100μg / mL ampicillin, culture at OD at 37°C and 220rpm 600 After reaching 0.6-0.8, IPTG with a final concentration of 0.1 mM was added, and the cells were collected after 22 hours of induction culture at 20°C and stored at -80°C. The specific purification steps are as follows:
[0152] (1) Resuspend the cells that have been col...
Embodiment 3
[0164] Example 3. Application of ALA Synthetase Mutant Enzymes in ALA Synthesis
[0165] Inoculate 5 mL of LB liquid medium containing 100 μg / mL ampicillin with a single colony of the above-mentioned recombinant bacteria, and culture at 37° C. and 220 rpm for 12 hours. According to the initial OD of 0.05, transfer to a 250mL Erlenmeyer flask containing 50mL fermentation medium, culture at 37°C, 220rpm for 3h, add IPTG with a final concentration of 0.05mM, collect the fermentation broth after 24h of induction culture, and detect the concentration of ALA. The shake flask fermentation medium formula is: glucose 15g / L, yeast powder 2.0g / L, Na 2 HPO 4 12H 2 O 17.1g / L, KH 2 PO 4 3.0g / L, NaCl 0.5g / L, NH 4 Cl 1.0g / L, MgSO 4 2.0mM, CaCl 2 0.1mM, glycine 4g / L, adjust the pH to 7.0. The final concentration of ampicillin was 100 μg / mL. ALA detection and glucose analysis methods are described in the "Materials and Methods" section. The fermentation results of the recombinant ba...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com