Method for weakening hemB gene expression to increase yield of 5-aminolevulinic acid synthesized by escherichia coli
A technology of aminolevulinic acid and Escherichia coli, applied in the fields of metabolic engineering and microbial fermentation, can solve problems such as high cost, unsuitability for large-scale industrial production, etc., and achieve the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
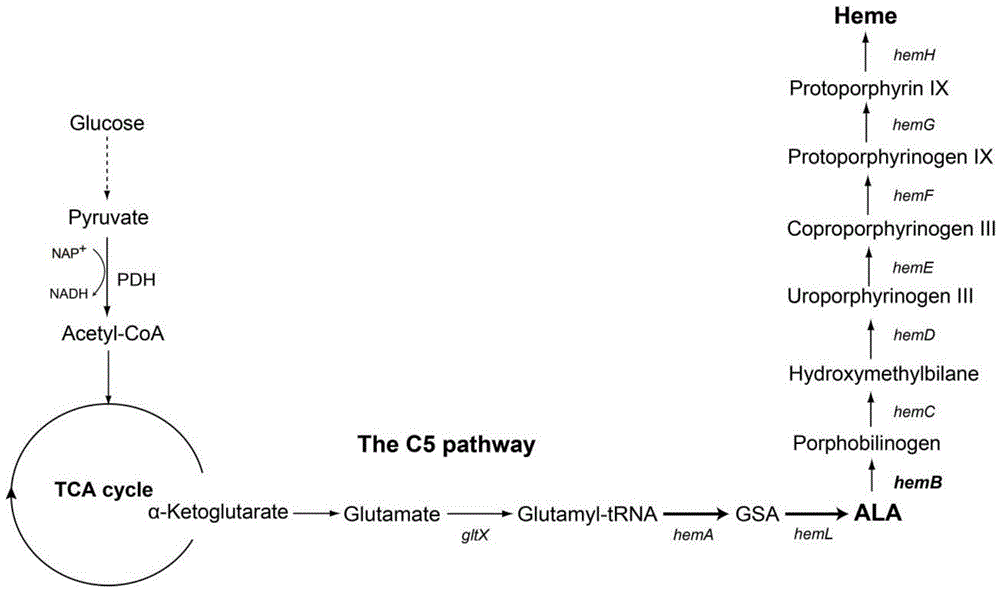
[0034] Example 1 Assembly of Homologous Recombination Fragment P1-Kan-PfliC-P2
[0035] Using the Escherichia coli genome as a template, the upstream homology arm P1 (934bp), the downstream homology arm P2 (726bp) and the fliC promoter PfliC (130bp) of the hemB gene promoter were respectively amplified, and the plasmid pKD13 was used as a template to amplify the selection marker anti- Sex gene Kan (1330bp). Then, four fragments were assembled by fusion PCR method, that is, P1-Kan-PfliC-P2 (3120bp).
[0036] Primers are as follows:
[0037] upstream homology arm P1
[0038] F1: CACTTGTATCAAATGTCTCATTTGTGTG
[0039] R1: CGAAGCAGCTCCAGCCTACACTTATTTATAGCTGTTGGTTATTATTTTTTGG
[0040] resistance gene Kan
[0041] F2: TAACCAACAGCTATAAATAAGTGTAGGCTGGAGCTGCTTCG
[0042] R2: TTTCAAAAACAGCCATTTTTTGATAAGCTGTCAAACATGAGAATTAATT
[0043] PromoterPfliC
[0044] F3: TCATGTTTGACAGCTTATCAAAAAATGGCTGTTTTTGAAAAAAATT
[0045] R3: CGTTGGATTAAGTCTGTCATGATTCGTTATTCCTATATTGCAAGTC
[0046] dow...
Embodiment 2
[0049] Example 2 HemB Gene Promoter Transformation
[0050] The promoter transformation method uses the Red recombination one-step knockout method:
[0051] (1) The plasmid pKD46 was transformed into E.coli BL21(DE3). The plasmid was temperature-sensitive, and the cell culture temperature was 30°C. Pick a single colony cultured in the slant medium and inoculate it in the SOB medium supplemented with 100mg / L ampicillin. After culturing overnight at 30°C for about 12 hours, transfer it to SOB culture supplemented with 100mg / L ampicillin at 2% inoculum Continue culturing in medium until OD600nm is about 0.1-0.2, add L-arabinose at a final concentration of 10mM to induce the expression of the recombinant enzyme, continue culturing until OD600nm is about 0.6, and bathe the bacterial solution in ice for about 10min. Centrifuge at 4000r / min for 10min at 4°C to collect the bacterial cells, then wash the bacterial cells three times with sterile 10% glycerol aqueous solution, concentra...
Embodiment 3
[0055] Embodiment 3 Recombinant bacteria fermentation verification
[0056] Strains:
[0057] LADF-6: E. coli BL21(DE3) / pRSFDuet-1-hemLA-hemFpETDuet-1-hemD.
[0058] PfliC-LADF-6:E.coli BL21(DE3)PfliC / pRSFDuet-1-hemLA-hemF pETDuet-1-hemD.
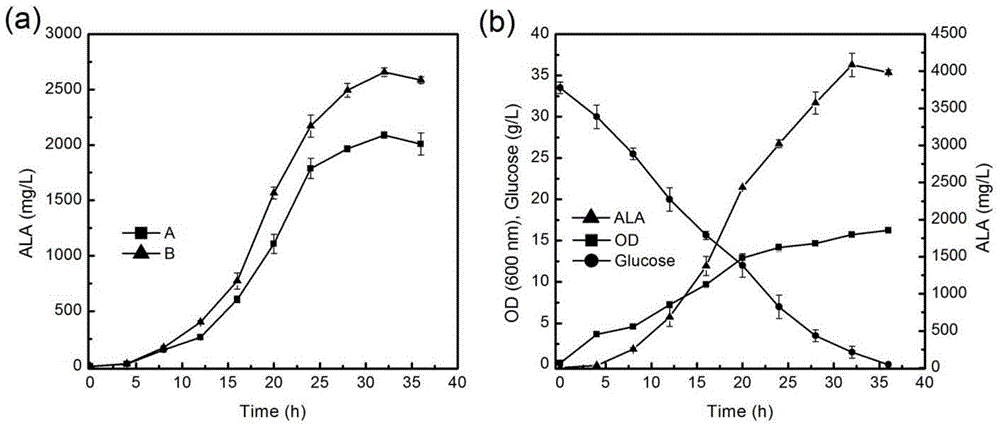
[0059] Recombinant Escherichia coli PfliC-LADF-6 and control bacterium LADF-6 were respectively fermented in 250mL Erlenmeyer flasks, the inoculum size was 1%, the initial glucose concentration was 20g / L, 0h added 0.1-0.5mM IPTG induction and ampicillin (100mg / L ) and kanamycin (50mg / L), ALA began to accumulate in large quantities after 10h, and the output was the highest at about 30h, which was 2680mg / L, which was increased by more than 25% compared with the control output ( image 3 a).
[0060] Recombinant Escherichia coli PfliC-LADF-6 was scaled up in a 3L fermenter with an inoculum size of 2% and an initial glucose concentration of about 35g / L. 0h was added with 0.1-0.5mM IPTG for induction and ampicillin (100mg / L) and kana Mycin (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com