Novel aminoketones photoinitiator and application in UV-LED photocuring system
A technology of photoinitiator and aminoketone, applied in organic chemistry, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of single UV-LED light source radiation peak, inability to provide initiating activity, limited absorption, etc., to achieve strong Intramolecular electron transfer performance, excellent photoelectric properties, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
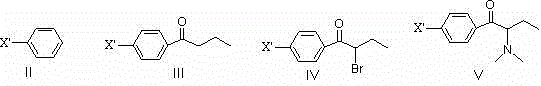
[0061] Embodiment 1: Preparation of intermediate 1-p-fluorophenyl-2-dimethylamino-2-benzyl-1-butanone
[0062] 1) Preparation of 1-p-fluorophenyl-1-butanone
[0063] Add 58.1g (0.44mol) of anhydrous aluminum trichloride, 34.9g (0.36mol) of fluorobenzene and 90mL of To 1,2-dichloroethane, slowly add 46.5g (0.44mol) of n-butyryl chloride below 10°C, and stir for 4-6h at 35-45°C. After the reaction solution is cooled to room temperature, pour it into hydrochloric acid-ice water, stir for 30 minutes, let it stand for layers, separate the organic phase, and wash the organic phase with sodium hydroxide solution (to remove n-butyric acid in the system), and then wash the organic phase once with water , solvent removal, and solvent recovery to obtain 1-p-fluorophenyl-1-butanone.
[0064] 2) Preparation of 1-p-fluorophenyl-2 bromo-1-butanone
[0065] Add 80mL of 1,2-dichloroethane, 36.9g of concentrated sulfuric acid and the prepared 1-p-fluorophenyl-1-butanone into a 500mL vessel ...
Embodiment 2
[0070] Example 2: Preparation of 1-p-chlorophenyl-2-dimethylamino-2-benzyl-1-butanone
[0071] 1) Preparation of 1-p-chlorophenyl-1-butanone
[0072] Add 46.1g (0.35mol) of anhydrous aluminum trichloride, 31.5g (0.28mol) of chlorobenzene and 70mL of To 1,2-dichloroethane, slowly add 36.8g (0.35mol) of n-butyryl chloride below 10°C, and stir for 4-6 hours at 35-45°C. After the reaction solution is cooled to room temperature, pour it into hydrochloric acid-ice water, stir for 30 minutes, let it stand for layers, separate the organic phase, and wash the organic phase with sodium hydroxide solution (to remove n-butyric acid in the system), and then wash the organic phase once with water , solvent removal, and solvent recovery to obtain 1-p-chlorophenyl-1-butanone.
[0073] 2) Preparation of 1-p-chlorophenyl-2 bromo-1-butanone
[0074] Add 65mL of 1,2-dichloroethane, 28.8g of concentrated sulfuric acid and the prepared 1-p-chlorophenyl-1-butanone into a 500mL vessel equipped wit...
Embodiment 3
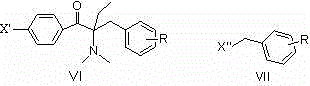
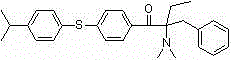
[0079] Example 3: Preparation of 1-[4-(4-isopropylphenylthio)phenyl]-2-dimethylamino-2-benzyl-1-butanone
[0080] 1) Using 1-p-fluorophenyl-2-dimethylamino-2-benzyl-1-butanone as raw material
[0081] In a 250mL four-neck flask equipped with a mechanical stirrer, a thermometer, a constant pressure dropping funnel, and a spherical reflux condenser, add 80mL of N,N-dimethylformamide solution dissolved in 2.8g (0.050mol) of potassium hydroxide , Slowly add 7.6g (0.050mol) p-isopropylthiophenol dropwise under stirring, and stir at room temperature for 1h. Add 10.0g (0.033mol) of 1-p-fluorophenyl-2-dimethylamino-2-benzyl-1-butanone, stir and raise the temperature to 100~120℃, and react for 10~15h. Cool the reaction solution, slowly add it dropwise to a large amount of ice water, then extract it several times with a small amount of ethyl acetate, separate the organic phase, wash the organic phase with saturated sodium chloride solution several times, dry over anhydrous sodium sulfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com