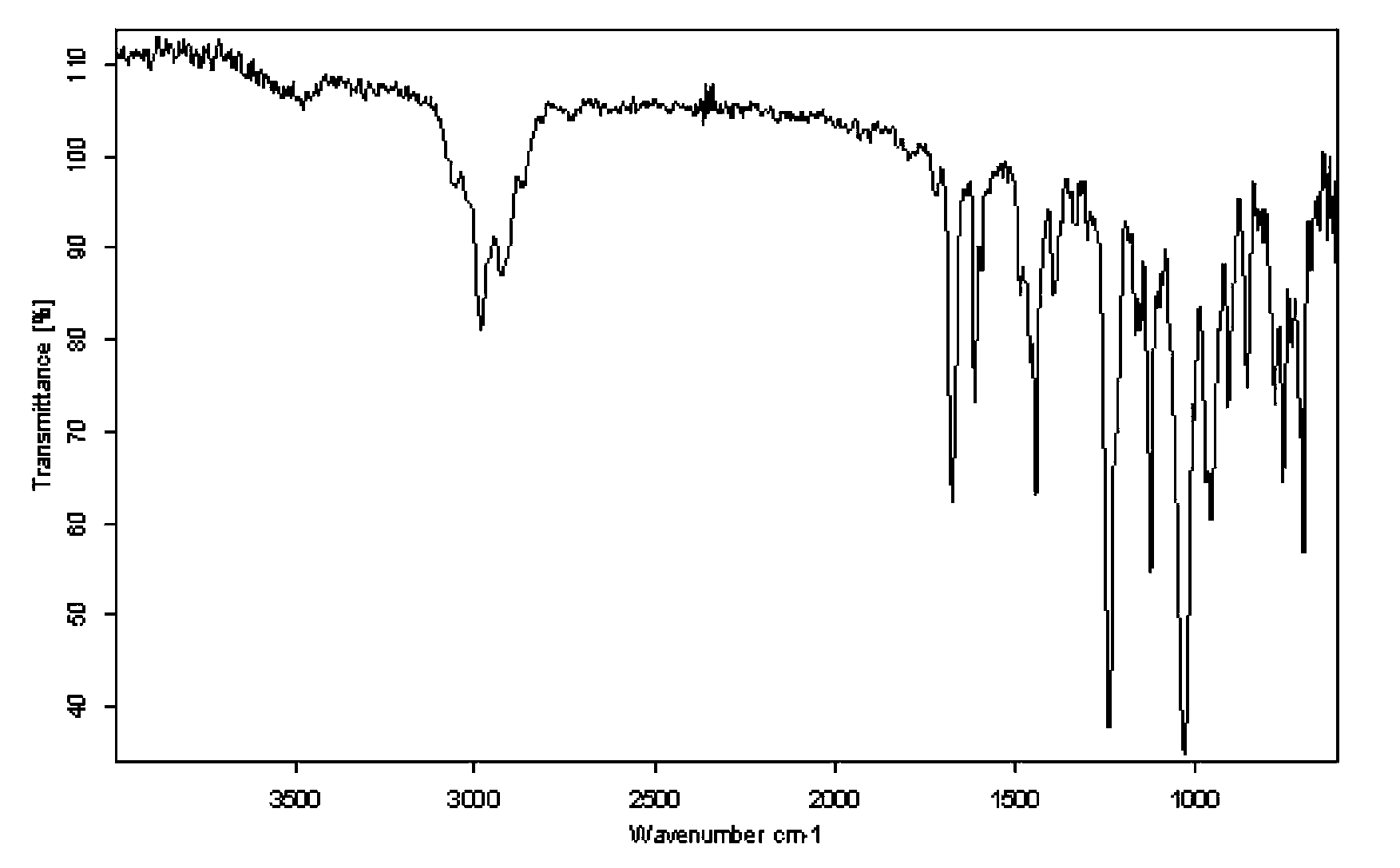
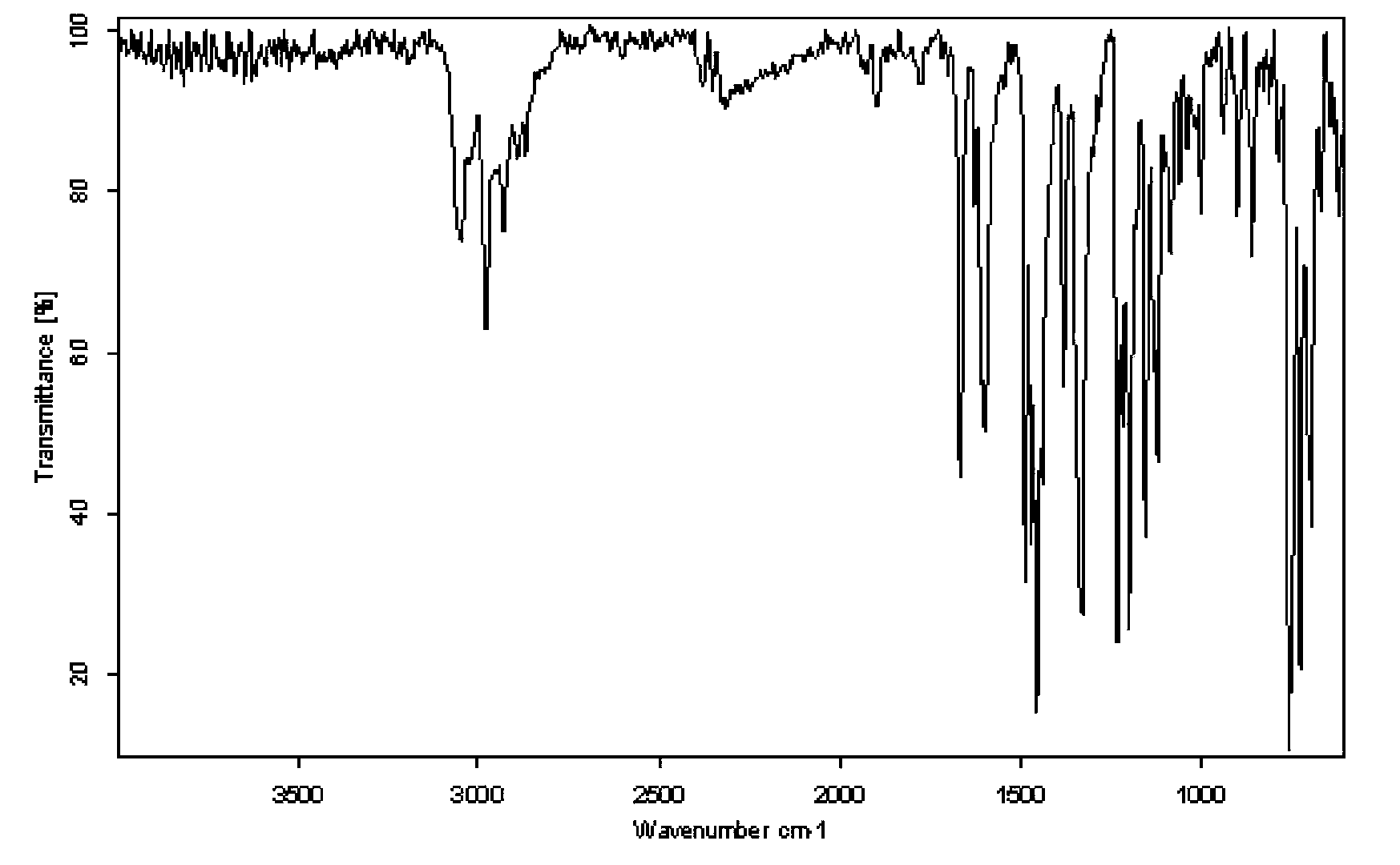
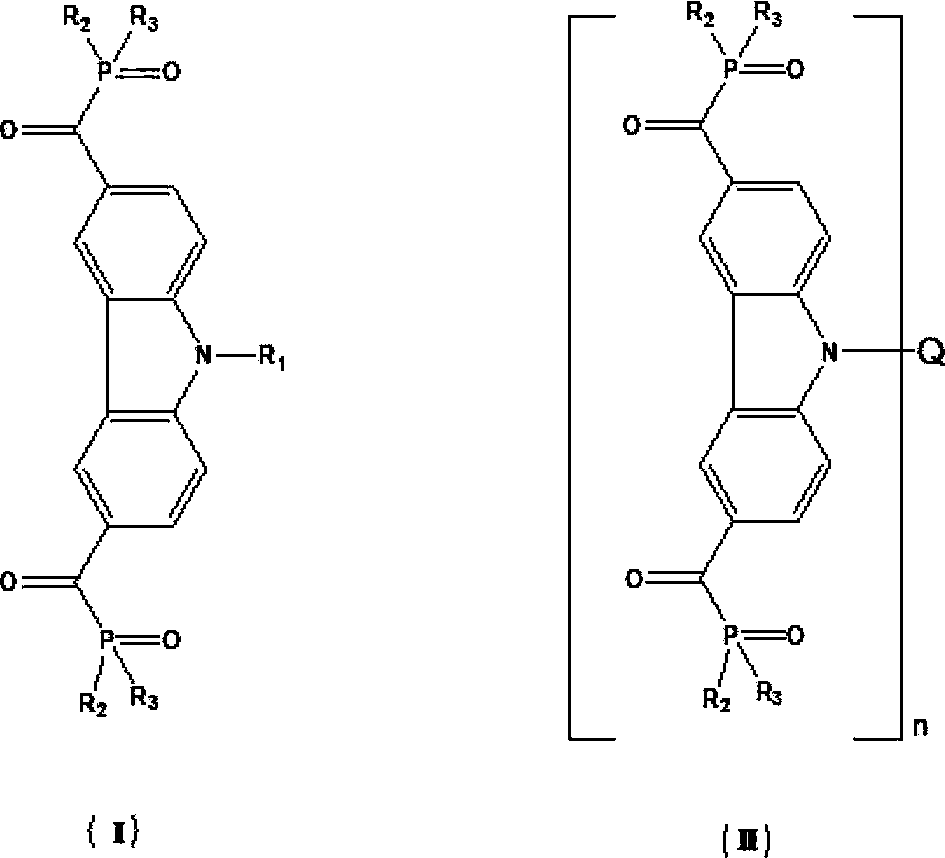
Photoinitiator for UV (ultraviolet)-LED curing
A UV-LED and photoinitiator technology, which is applied in the field of UV-LED curing photoinitiator and its preparation, can solve the problems of limited selection of curing substrates, many synthesis steps, and poor product stability. The effect of strong intramolecular electron transfer performance, good electron delocalization, and excellent photoelectric properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Embodiment 1: Preparation of formula (III) compound PI6301
[0075] Step 1: Carbazole N-ethylation
[0076] Add 20g of carbazole, 30ml of benzene, 60ml of 30% sodium hydroxide solution, and 1g of tetrabutylammonium bromide into a 250ml three-necked flask, and control the temperature at 50°C for 3 hours, then slowly add 14.3g of bromoethane dropwise, and reflux for 5 hours . TLC followed the reaction process. After the reaction was completed, 70ml of benzene and 50ml of water were added, stirred and allowed to stand for stratification. The organic phase was washed twice with 50ml of water, and recrystallized with ethyl acetate after solvent removal to obtain 20.5g of N-ethylcarbazole. , yield 87.9%.
[0077] The second step: carboxylation
[0078] (a) With reference to the literature Inorganic Chemistry Communications 9(2006) 351–354, AlCl 3 (40g, 0.3mol) and acetyl chloride (35ml, 0.5mol) were dissolved in 25ml of dichloromethane, at normal temperature, the dichloro...
Embodiment 2
[0086] Embodiment 2: the preparation of formula (IV) compound PI6302
[0087] Referring to Example 1, the first, second and third steps were used to prepare 3,6-diacetyl chloride-9-ethylcarbazole;
[0088] The fourth step: the preparation of diethyl phenyl phosphite
[0089] Under stirring, 179g (1.0mol) of phenylphosphine dichloride was slowly added dropwise to 266.6g (2.2mol) of N,N-dimethylaniline and 276g (0.6mol) of ethanol in an ice bath kept at 20-30 The mixture was stirred at room temperature for 3h after the addition. Filter off the solid salt, wash with ether several times, combine the filtrate and washing liquid, evaporate the solvent under normal pressure, and then distill under reduced pressure to collect 158.5 g of b.p.109°C / 1.59kpa fraction, with a yield of 80%.
[0090] Step 5: Arbuzov reaction
[0091] Place 31 g of the obtained 3,6-diacetyl chloride-9-ethylcarbazole in a 250 ml three-necked flask, slowly add 38 g of phenyl diethyl phosphite dropwise under ...
Embodiment 3
[0093] Embodiment 3: Preparation of formula (XIII) compound PI6401
[0094] The first step: the preparation of 1,6-biscarbazolidine
[0095] Add 20g of carbazole, 30ml of benzene, 60ml of 30% sodium hydroxide solution, and 1g of tetrabutylammonium bromide into a 250ml three-necked flask, and slowly add 16.5g of 1,6-dibromohexane dropwise after controlling the temperature at 50°C for 3 hours. , Reflux reaction for 5h. TLC followed the reaction process. After the reaction was completed, 70ml of benzene and 50ml of water were added, stirred and allowed to stand for stratification, and the organic phase was washed twice with 50ml of water. After removing the solvent, it was recrystallized with ethyl acetate to obtain 1,6-biscarbazole hexyl Alkane 18g, yield 71%.
[0096] The second step: carboxylation of 1,6-biscarbazole hexane
[0097] (a) With reference to the literature Inorganic Chemistry Communications 9(2006) 351–354, AlCl 3 (40g, 0.3mol) and acetyl chloride (33.6ml, 0.4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com