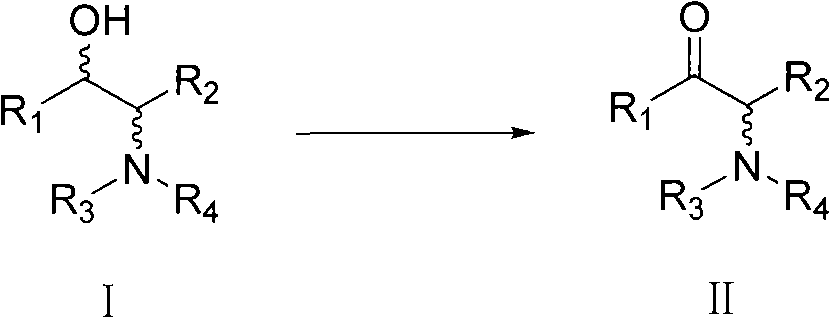
Preparation method for alpha-amino ketone derivative
A technology of aminoketone and derivatives, which is applied in the field of preparation of α-aminoketone derivatives, and can solve the problems of too strong oxidation of reagents, serious side reactions, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] 4.042g (19.5mmol) of 2-(N-acetyl)methylamino-1-phenyl-1-propanol was dissolved in 30mL of ethyl acetate, and under stirring, 0.031g (0.2mmol, 0.01eq. ) 2,2,6,6-tetramethylpiperidine-1-oxyl radical, then slowly dropwise add 1.526g (6.58mmol, 0.34eq.) 10mL ethyl acetate solution of TCCA, after adding, stir at room temperature React for 30 minutes. After the reaction was completed, it was washed twice with 30 mL of saturated sodium bisulfite solution and 30 mL of distilled water successively, and concentrated. 3.888 g of the concentrate was obtained. The calculated yield was 97.12%. After identification by HPLC, it was known that the product was 2-(N-acetyl)methylamino-1-phenyl-1-propanone, and the HPLC purity was 99.5%.
Embodiment 2
[0084] 4.501g (21.7mmol) of 2-(N-acetyl)methylamino-1-phenyl-1-propanol was dissolved in 30mL of toluene, and under stirring, 0.036g (0.23mmol, 0.01eq.)2 was added to the system , 2,6,6-tetramethylpiperidine-1-oxyl radical, then slowly dropwise add 1.543g (6.65mmol, 0.33eq.) 10mL toluene solution of TCCA, after adding, stir and react at room temperature for 30 minutes . After the reaction was completed, it was washed twice with 30 mL of saturated sodium bisulfite solution and 30 mL of distilled water successively, and concentrated. 3.748 g of the concentrate was obtained. The calculated yield was 84.08%. The HPLC purity of the product in the reaction solution was 94.37%.
Embodiment 3
[0086] 0.503g (2.4mmol) of 2-(N-acetyl)methylamino-1-phenyl-1-propanol was dissolved in 10mL of ethyl acetate, and under stirring, 0.004g (0.02mmol, 0.01eq. ) 2,2,6,6-tetramethylpiperidine-1-oxygen free radical, then slowly dropwise add 0.188g (0.8mmol, 0.33eq.) 5mL ethyl acetate solution of TCCA, after adding, at 40°C The reaction was stirred for 30 minutes. After the reaction was completed, it was washed twice with 15 mL of saturated sodium bisulfite solution and 15 mL of distilled water successively, and concentrated. 0.483 g of the concentrate was obtained. The calculated yield was 96.97%. The HPLC purity of the product in the reaction solution was 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com