Patents
Literature
38 results about "Istradefylline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
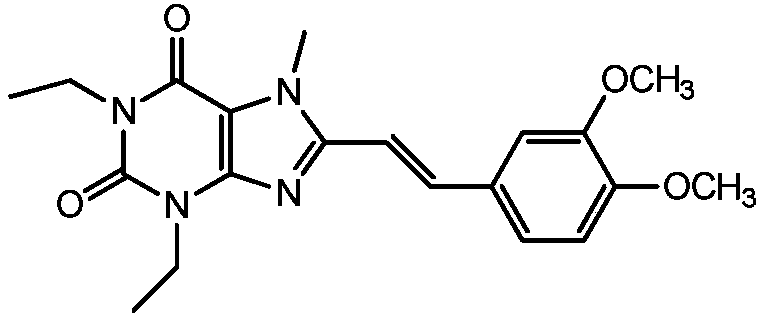
Istradefylline, sold under the brand name Nourianz, is a medication used to treat Parkinson's disease (PD). Istradefylline reduces "off" periods resulting from long-term treatment with the antiparkinson drug levodopa. An "off" episode is a time when a patient’s medications are not working well, causing an increase in PD symptoms, such as tremor and difficulty walking.
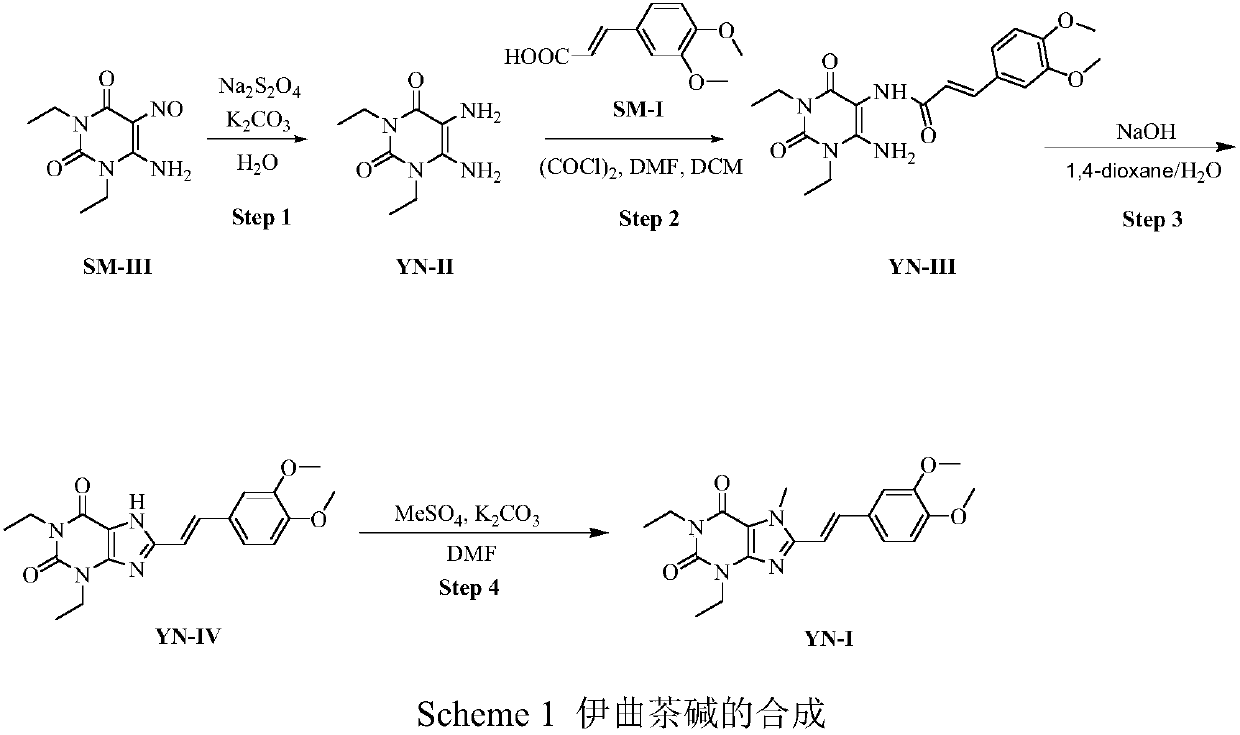
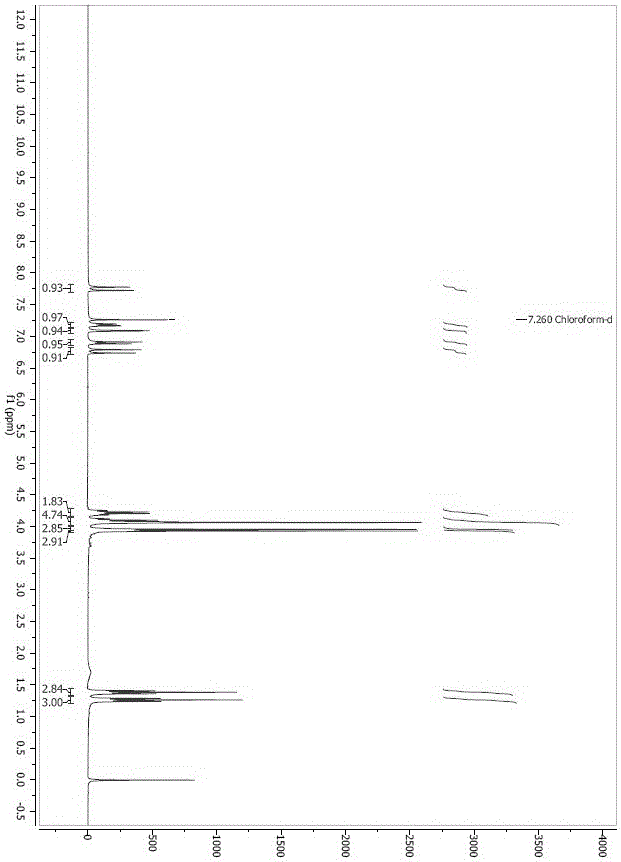
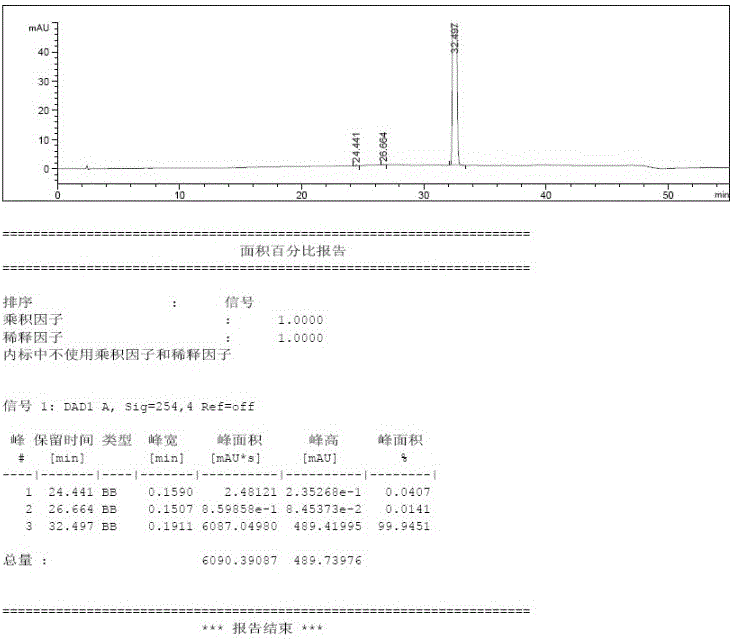
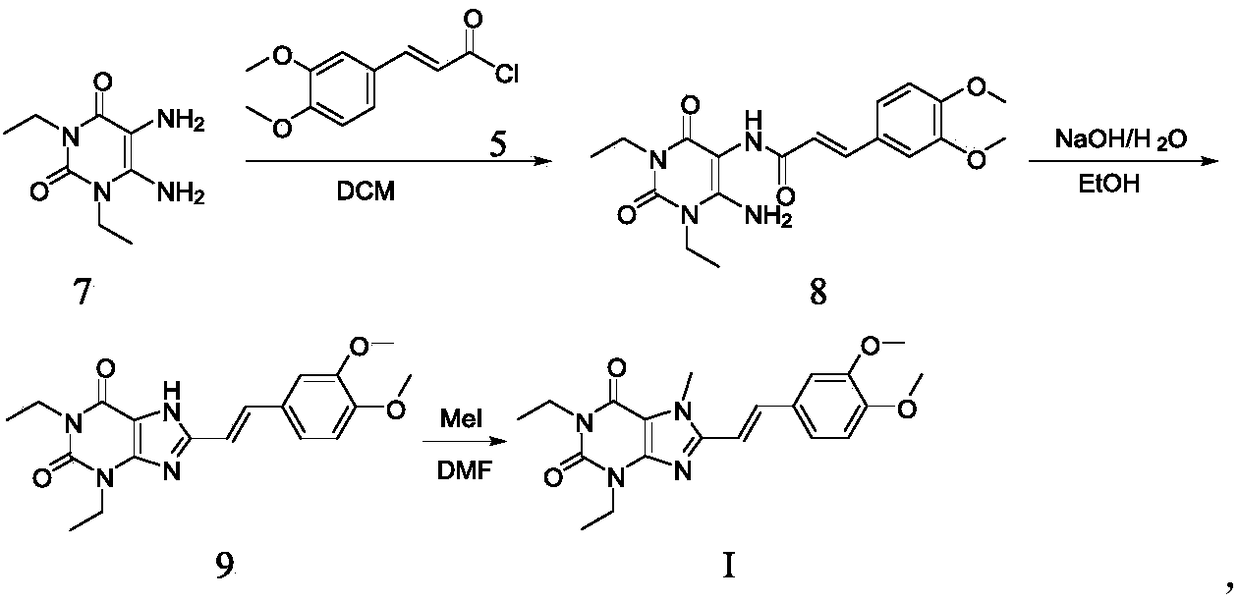
Istradefylline synthesis process
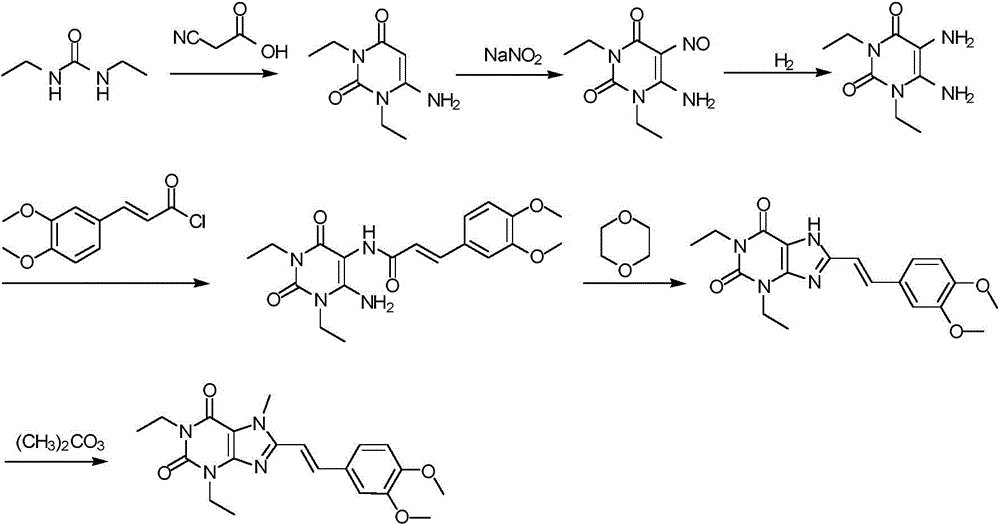
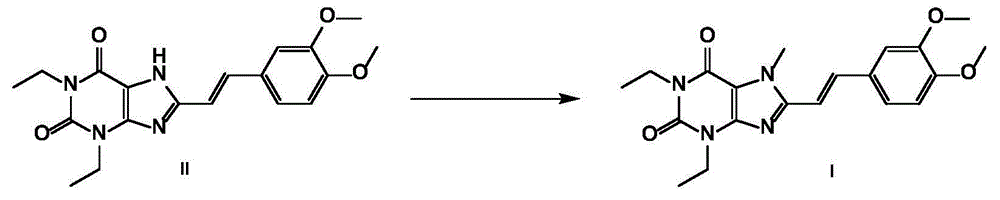
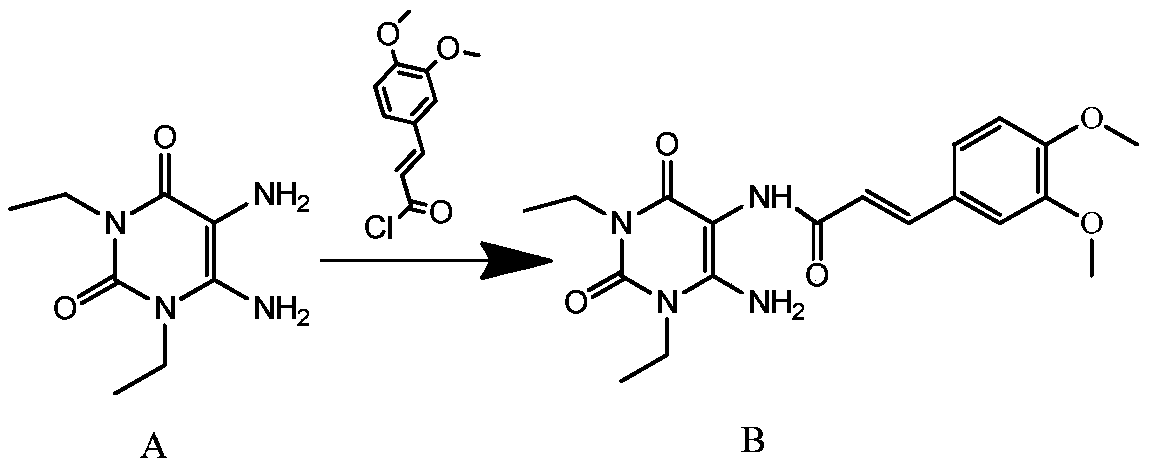
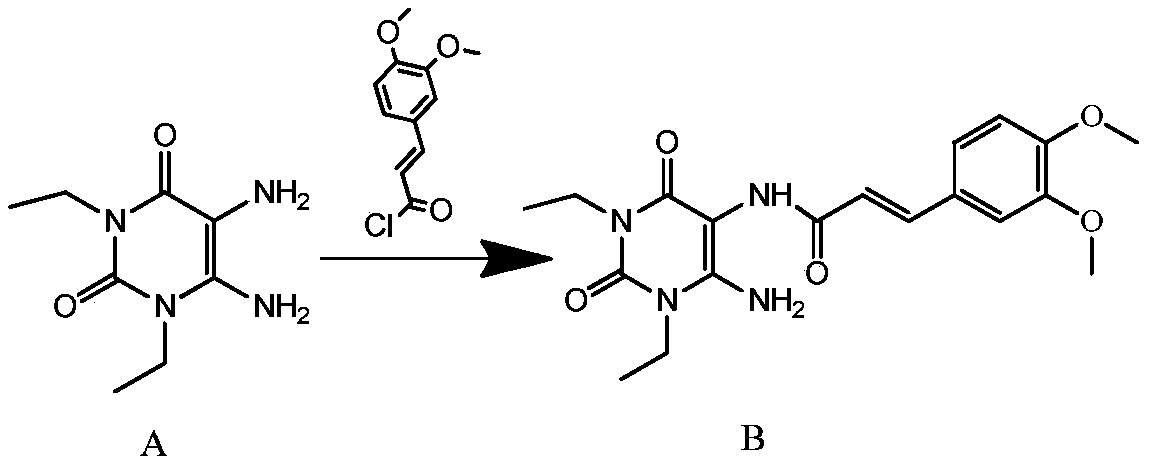
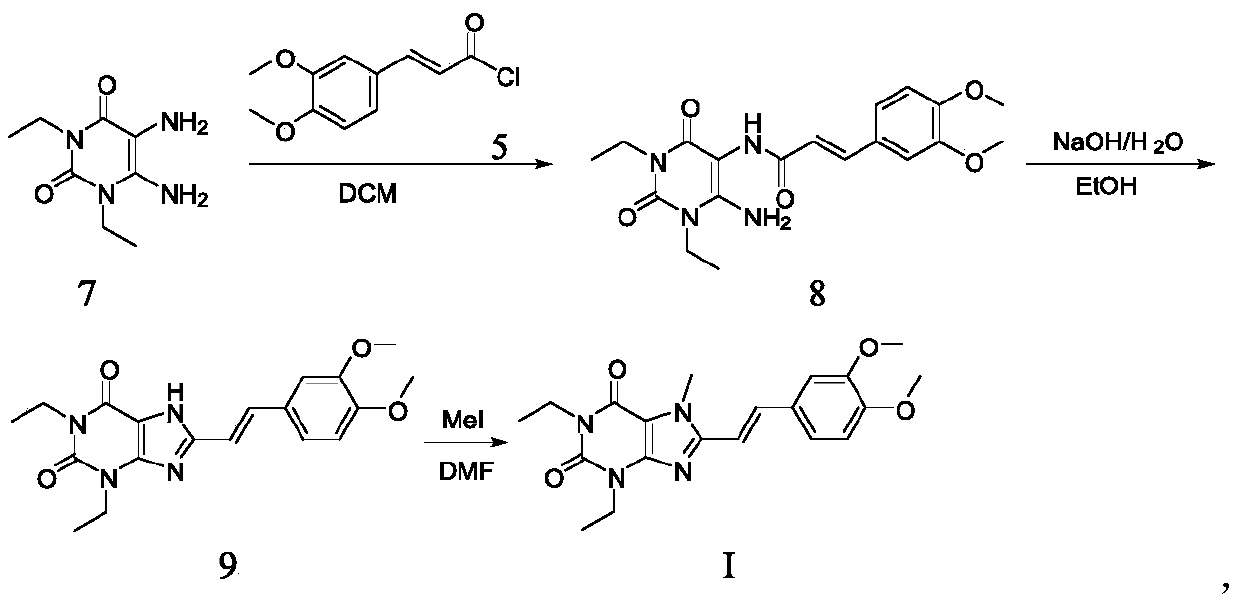
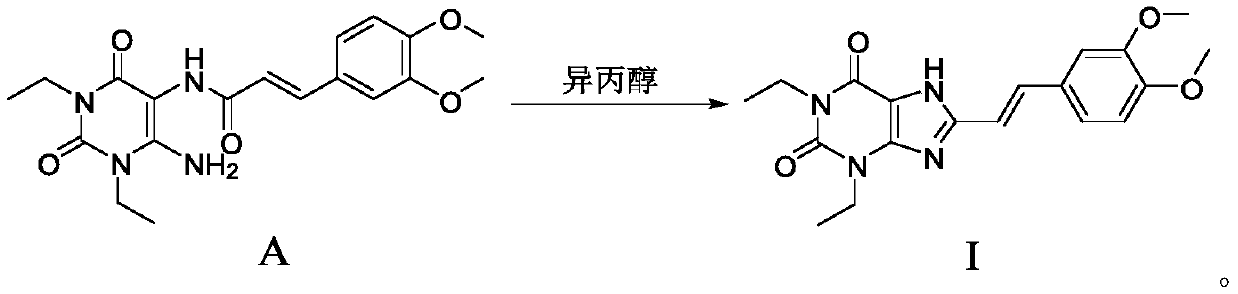
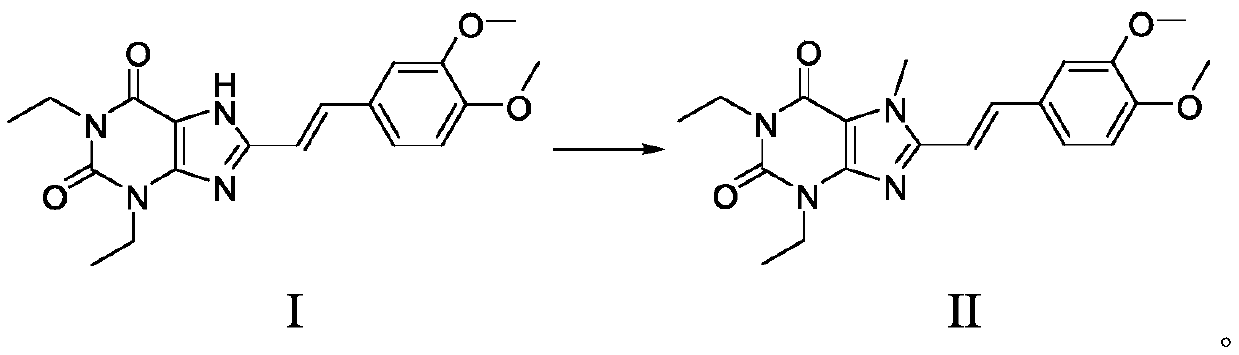
The invention discloses an istradefylline synthesis process comprising the following steps: a compound I is prepared from 1,3-diethylformamide and cyanoacetic acid in acetic anhydride; the compound I is added into acetic acid, and the mixture is stirred for 30min; sodium nitrite is added, and thus a compound II is prepared; hydrogen gas is introduced into the compound II and methanol, such that a compound III is obtained; the compound III, 3-(3,4-dimethoxyl-phenol)-acryloyl chloride, sodium hydroxide and dichloromethane are prepared into a compound IV; the compound IV and sodium hydroxide are added into 1,4-dioxane, such that a compound V is prepared; the compound V, dimethyl carbonate, DMF, and sodium hydroxide are subjected to a methylation reaction; and cooling, suction filtration and washing are carried out, such that istradefylline is obtained. With the process, the final product can be obtained with high efficiency; the synthesis route is simple; purification is easy; the application of a highly toxic or neurotoxic methylation reagent, such as iodomethane, is avoided. The process is suitable for industrialized productions, and assists in reducing the production cost.
Owner:NANJING CORE TECH CO LTD
Preparation method of istradefylline intermediate
ActiveCN104262342AImprove qualityHigh temperature and high qualityOrganic chemistryIstradefyllineUracil
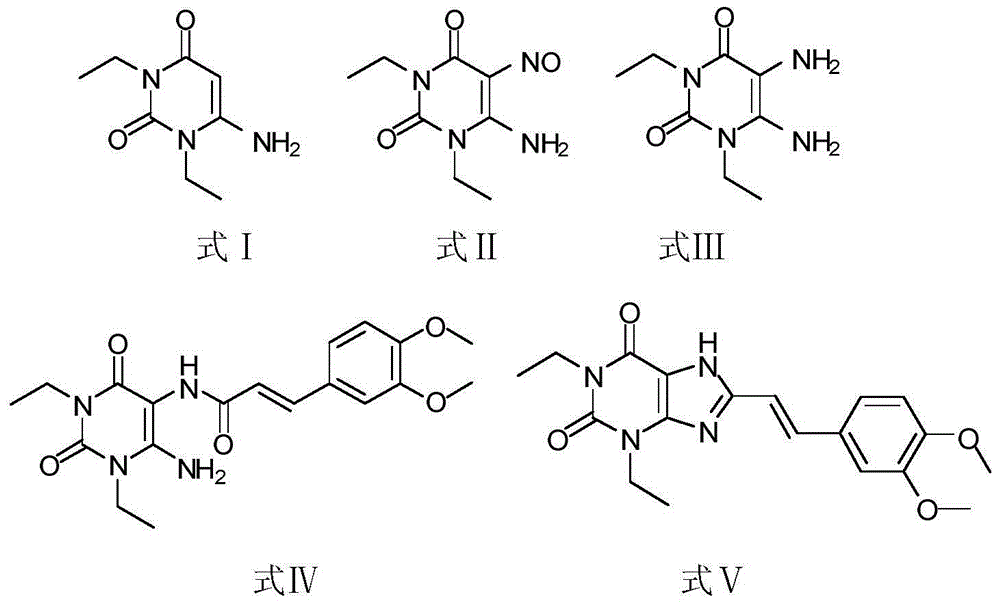
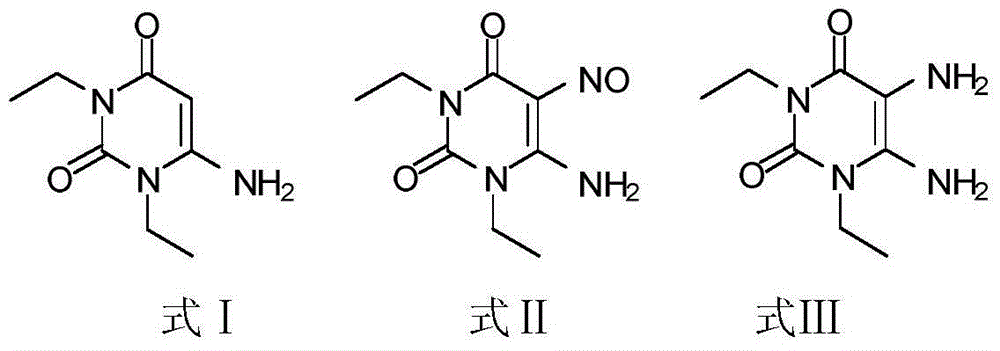
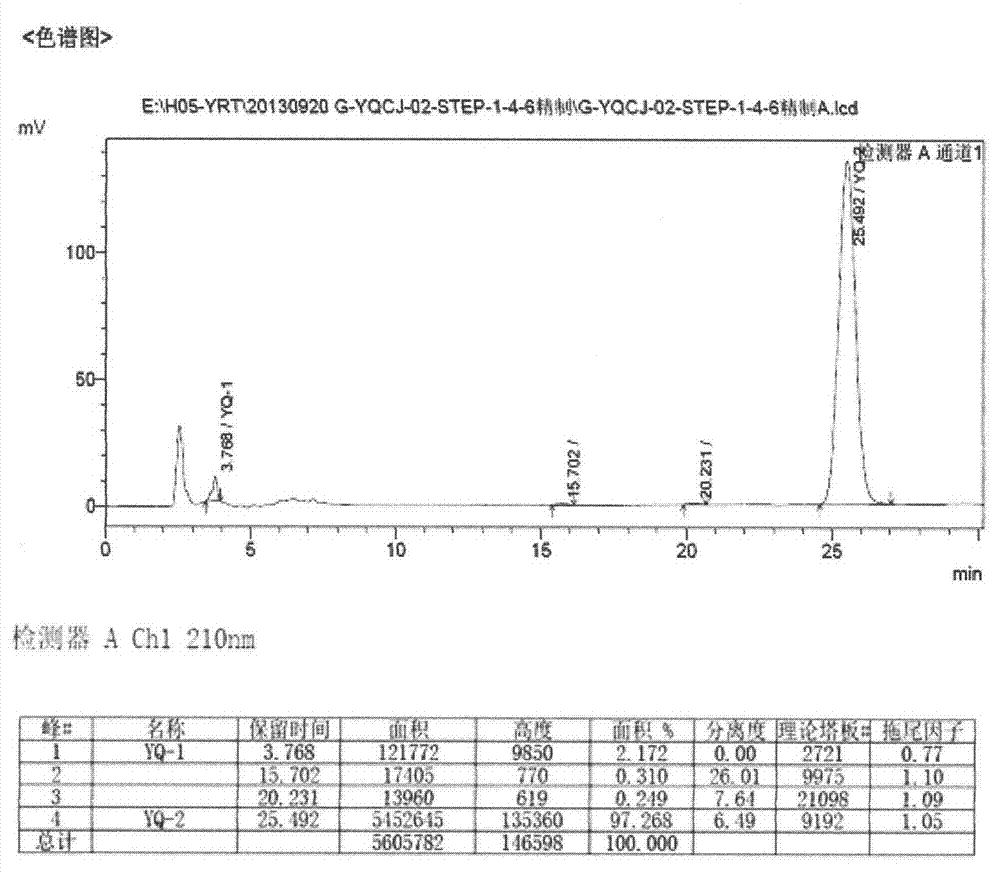
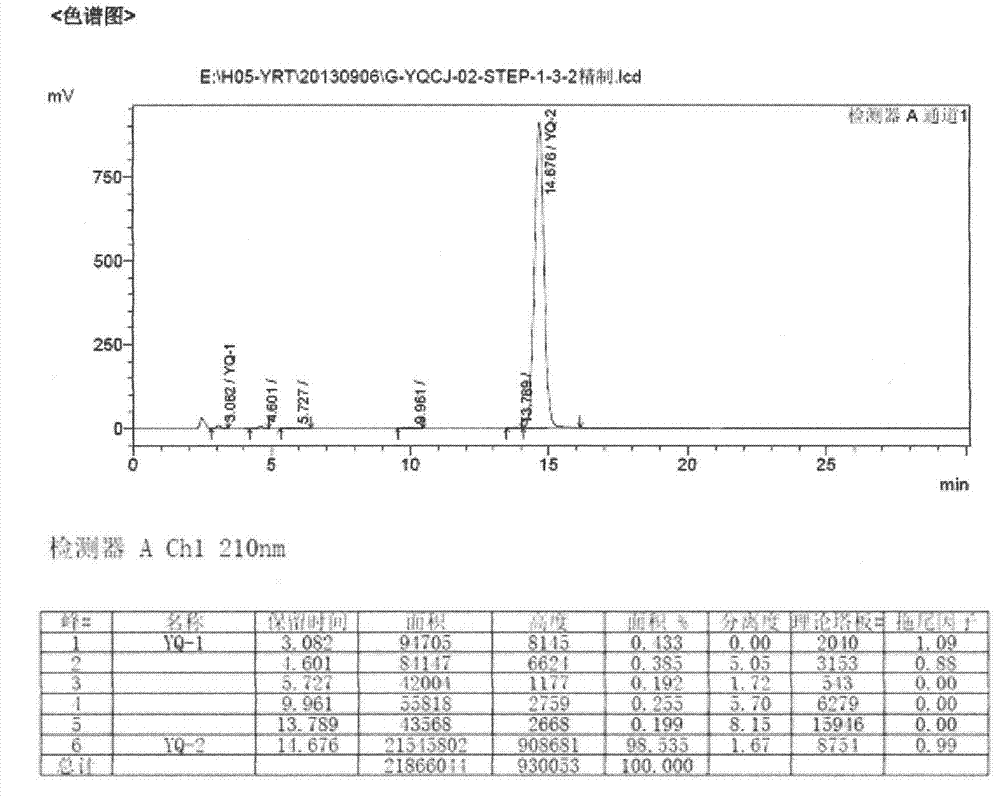
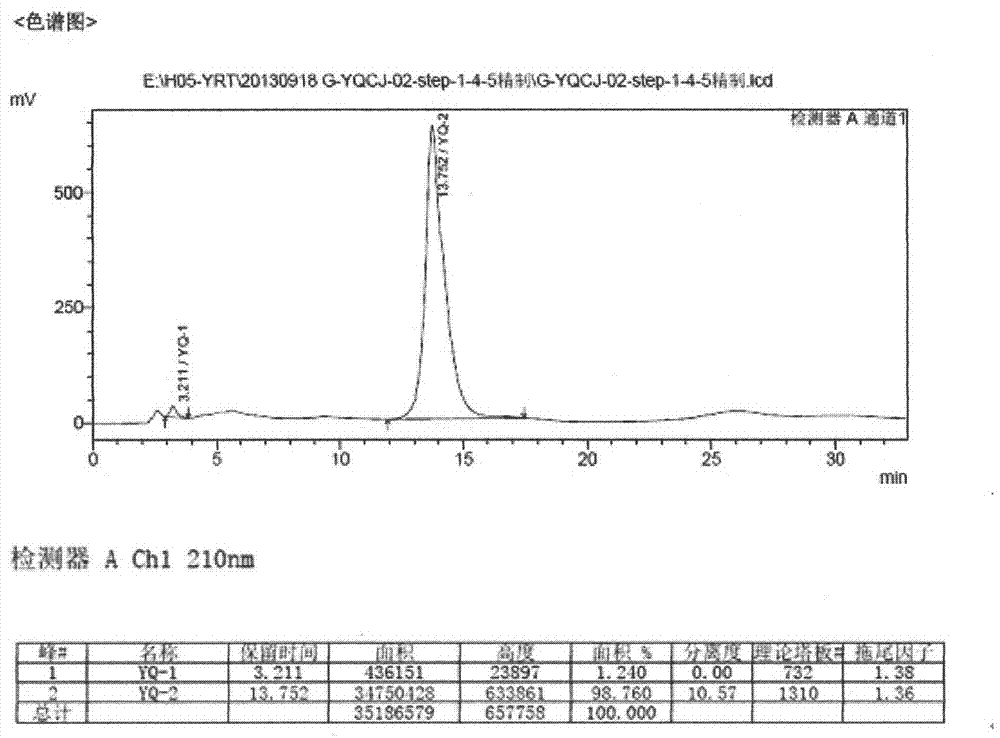
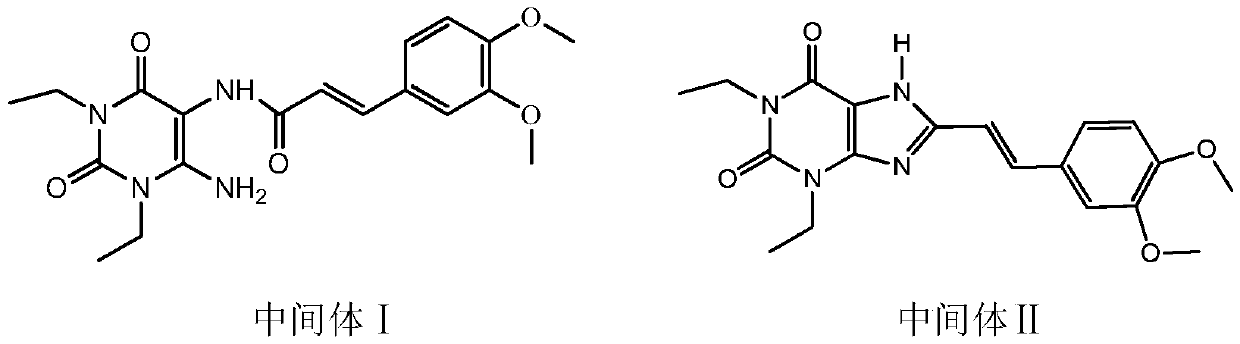
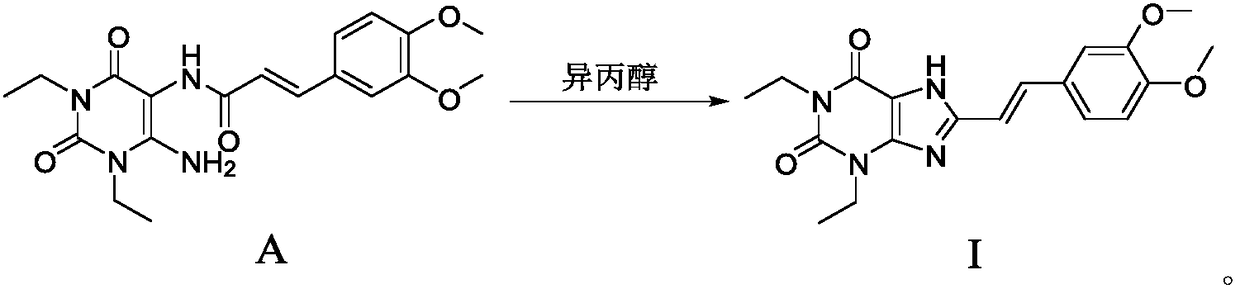
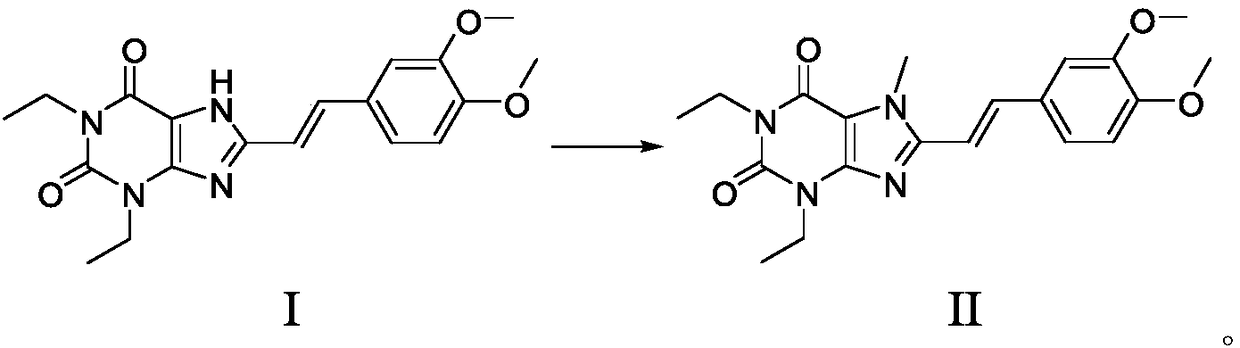
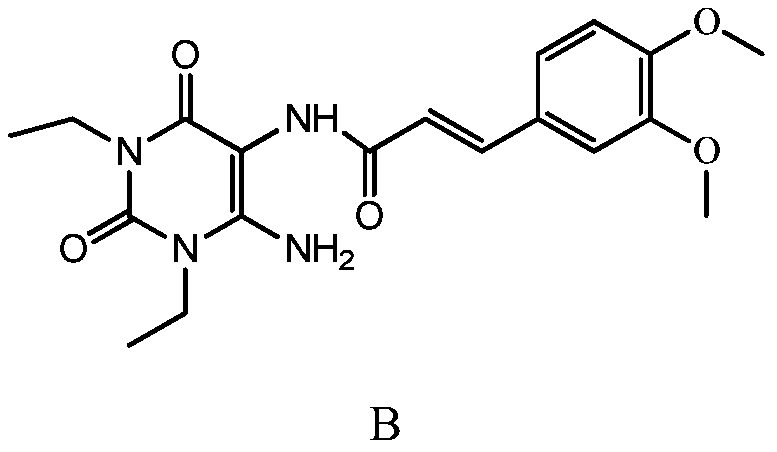
The invention relates to a preparation method of an istradefylline intermediate, which comprises the following steps: by using (E)-1,3-diethyl-6-amino-5-(3,4-dimethoxyphenylacryloyl)amino-uracil (YQ-1) as a raw material and 1,4-dioxane as a solvent, heating under the alkaline condition of a NaOH water solution to perform condensation and cyclization to generate (E)-8-[2-(3,4-dimethoxyphenyl)vinyl]-1,3-diethyl-3,7-dihydro-1H-purinyl-2,6-dione (YQ-2), and carrying out rotary evaporation, acid regulation and vacuum filtration to obtain a YQ-2 crude product; and by using toluene as a solvent, recrystallizing the YQ-2 crude product to obtain the YQ-2 refined product. The method has the advantages of simple route, accessible raw materials and mild conditions, and is convenient to operate; the total yield is greater than 85%, and the purity is up to higher than 97%; and thus, the method is suitable for scale-up production.
Owner:南京恒道医药科技股份有限公司
New crystal form of istradefylline and preparation method thereof
ActiveCN106279169AImprove stabilityGood crystal formNervous disorderOrganic chemistry methodsIstradefyllineX-ray
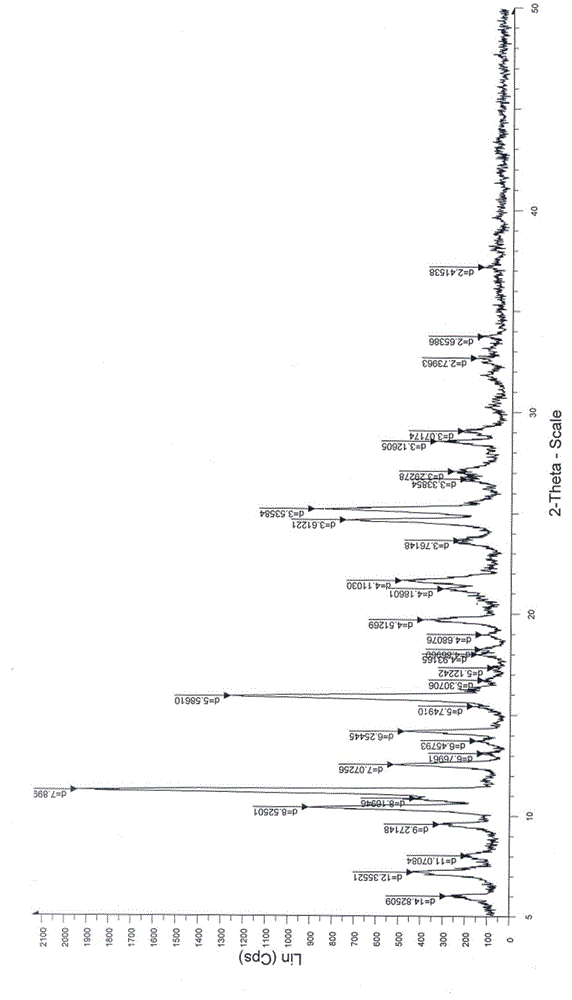
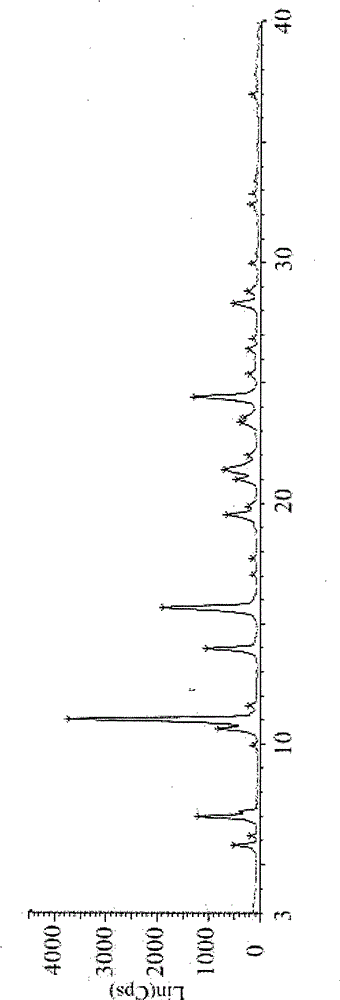
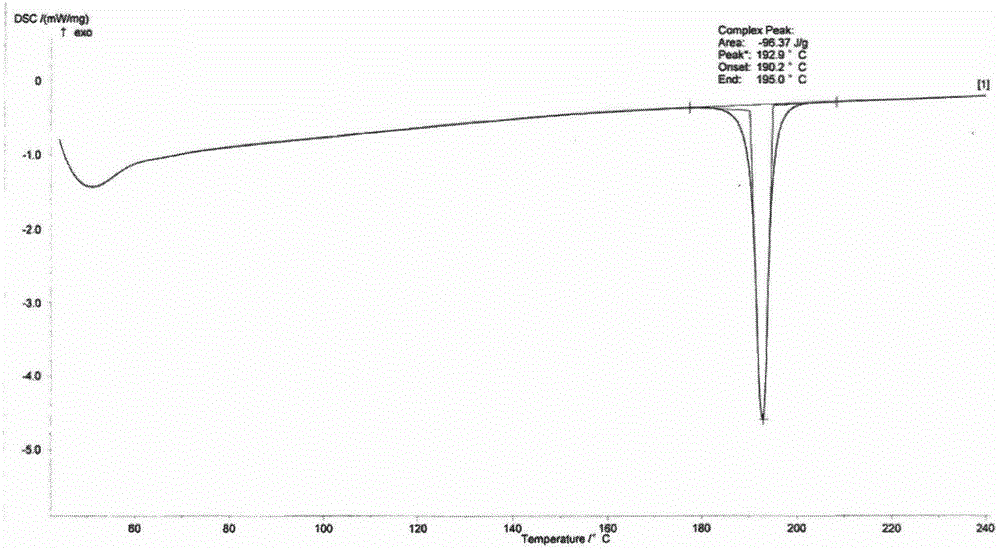
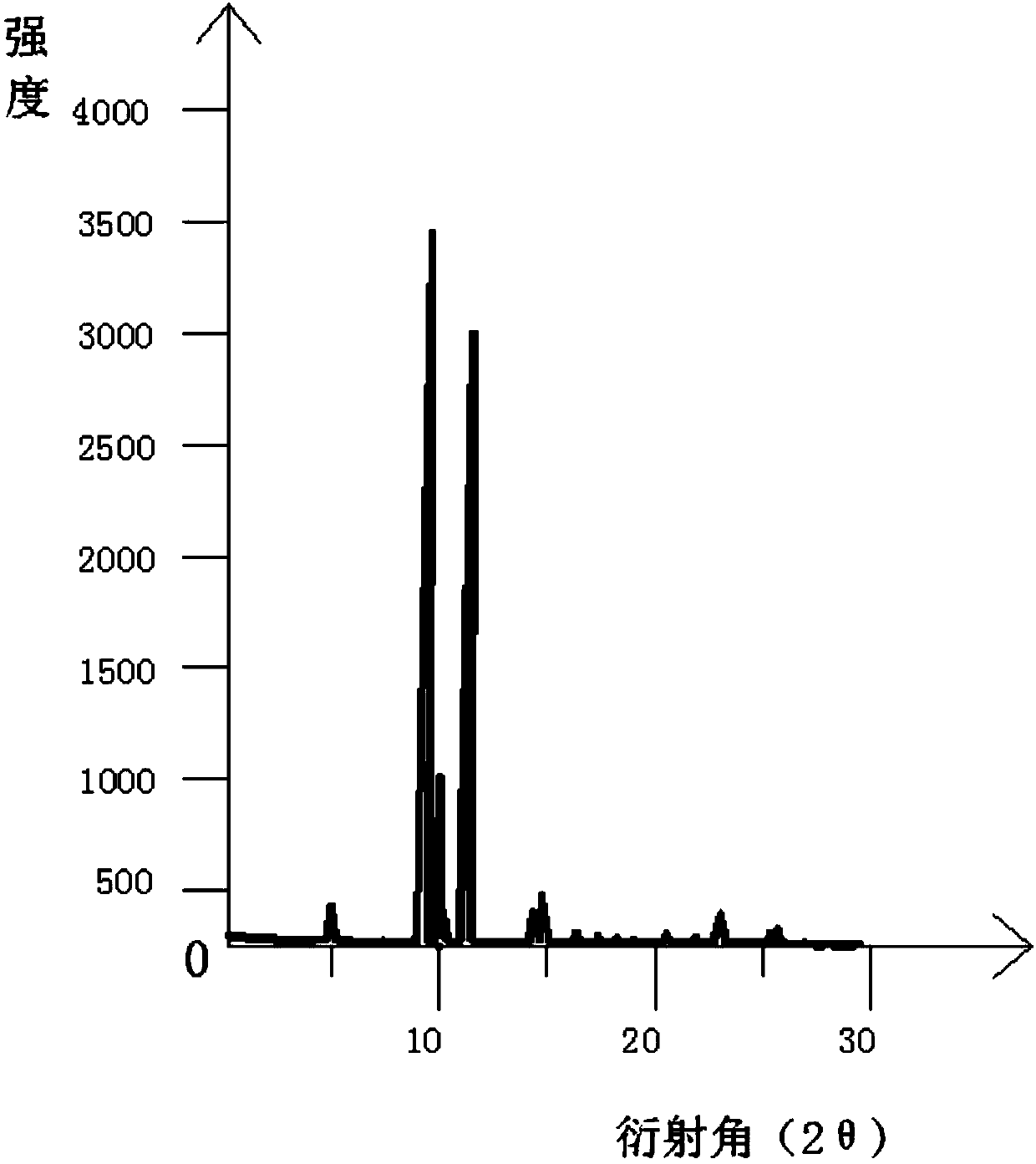
The invention discloses a new crystal form of istradefylline and a preparation method thereof. The powder X-ray diffraction of the new crystal form of istradefylline has characteristic peaks where 2theta is equal to 10.4 + / -0.2 degrees, 11.2 + / -0.2 degrees, 15.8 + / -0.2 degrees, 24.6 + / -0.2 degrees, and 25.2 + / -0.2 degrees. The new crystal form of istradefylline is thermodynamically stable and has obviously improved solubility in water; the new crystal form of istradefylline, which is crystalline solid powder having a small particle size, is good in fluidity, so that the problem of non-uniform crystal condensation and dispersion in process operation of a preparation can be solved; the new crystal form of istradefylline is more suitable for storage and for use as a bulk pharmaceutical chemical; and a new way is provided for the preparation of istradefylline drugs.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
New crystal form of istradefylline and preparation method thereof
The invention relates to a new crystal form of istradefylline and a preparation method thereof; the new crystal form IV has a powder X-ray diffraction pattern having main characteristic absorption peaks at diffraction angles (2theta) of 5.763 degrees, 6.958 degrees, 10.599 degrees, 10.999 degrees, 13.944 degrees, 15.647 degrees, 19.513 degrees, 21.004 degrees, 21.400 degrees, 24.426 degrees and 28.320 degrees. The invention also discloses the preparation method of the new crystal form IV which has simple preparation process and relatively good stability and conforms to medicinal requirements.
Owner:南京瑞天医药科技有限公司
Preparation method and use of istradefylline
InactiveCN106496227AIncrease productivityReduce manufacturing costOrganic chemistryIstradefyllineCyanoacetic acid
The invention relates to the technical field of pharmaceutical chemistry, and particularly relates to a preparation method and use of istradefylline; specifically, with 1,3-diethyl formamide and cyanoacetic acid as raw materials, istradefylline is prepared through cyclization, nitration, reduction, condensation, cyclization, methylation reaction and other steps; the preparation method provided by the invention is simple, high in production efficiency and wide in application prospect.
Owner:合肥美利康医药技术股份有限公司
Orally disintegrating tablet containing Istradefylline and preparation method thereof
InactiveCN105534928ANervous disorderPharmaceutical non-active ingredientsPharmacy technologyPharmacy
The invention discloses an orally disintegrating tablet containing Istradefylline and a preparation method thereof. The tablet contains a water-soluble filler, a binder, a disintegrating agent, a lubricant, and an odor mask. A solid dispersion technology is employed to improve the taste. The tablet has the characteristics of good taste, good oral compliance, rapid onset, and is applicable to the treatment of Parkinson's disease. The invention also provides a preparation method. The method has simple process and low cost, and belongs to the medical technical fields of medical science and pharmacy.
Owner:BEIJING VENTUREPHARM BIOTECH
Use of istradefylline for treating behavioral disorders
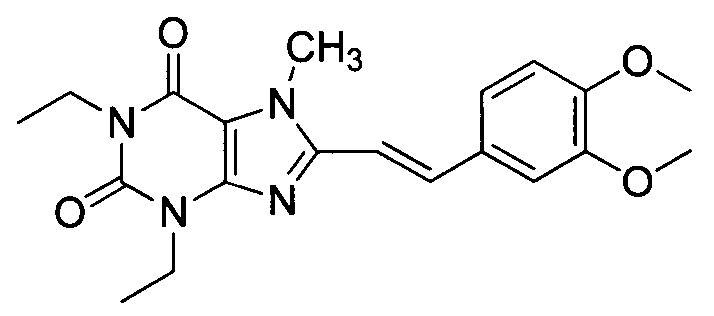
The present invention provides a method of treating behavioral disorders such as attention deficit hyperactivity disorder, comprising administering an effective amount of (E)-8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methylxanthine or a pharmaceutically acceptable salt thereof to a patient in need thereof and the like.
Owner:KYOWA HAKKO KOGYO CO LTD
Istradefylline preparation method
The invention provides an istradefylline preparation method. 1,3-diethyl-5,6-diaminouracil used as a starting material undergoes an acylation reaction, a ring formation reaction and a methylation reaction to prepare istradefylline. The ring formation reaction and the methylation reaction are carried out simultaneously, an istradefylline intermediate (E)-1,3-diethyl-6-amino-5-(3,4-dimethoxyphenylacryloyl)aminouracil is reacted by adding a methylating agent in an alkaline environment, and the obtained solution is filtered and crystallized to obtain the istradefylline. The ring formation and themethylation are simultaneously carried out, and the methylation is immediately carried out after the completion of the ring formation to promote the forward proceeding, so the yield of the product isincreased, large amounts of organic solvents are saved, and the generation amount of an organic waste liquid is reduced; and the method has the advantages of simplicity in operation, and high reactionconversion rate, and allows the purity of the product to be higher than 99%.
Owner:SHANDONG XINHUA PHARMA CO LTD
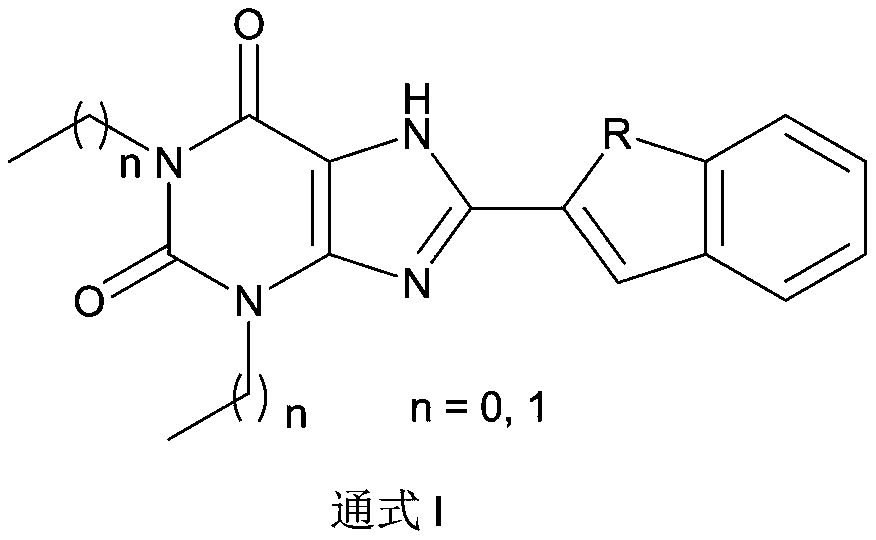
Xanthine derivative as well as preparation method and application thereof
The invention provides a xanthine derivative as well as a preparation method and application thereof, and particularly relates to application of the xanthine derivative serving as A2A receptor antagonists in preparation of medicines for treating adenosine receptor A2A related diseases. The xanthine derivative provided by the invention has better water solubility than istradefylline, and the bioavailability is greatly improved; the purpose of the present invention is to design and synthesize a novel xanthine derivative having good A2A receptor inhibitory activity. The prepared compound shows agood result in an A2A receptor activity test.
Owner:SHANDONG XINHUA PHARMA CO LTD
Improved synthesis technology of istradefylline
The invention provides an improved synthesis technology of istradefylline. According to the technology, istradefylline is prepared from 1,3-diethyl-6-amino-5-nitrosouracil (SM-III) as a starting material through four steps.
Owner:FUKANGREN BIO PHARMA
Novel preparation method for Istradefylline
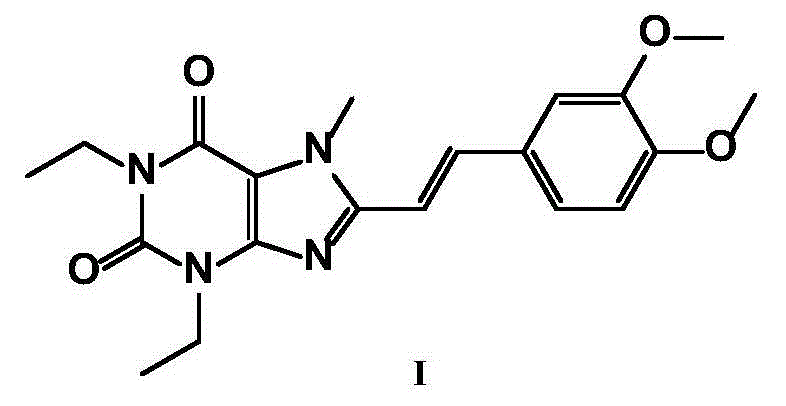
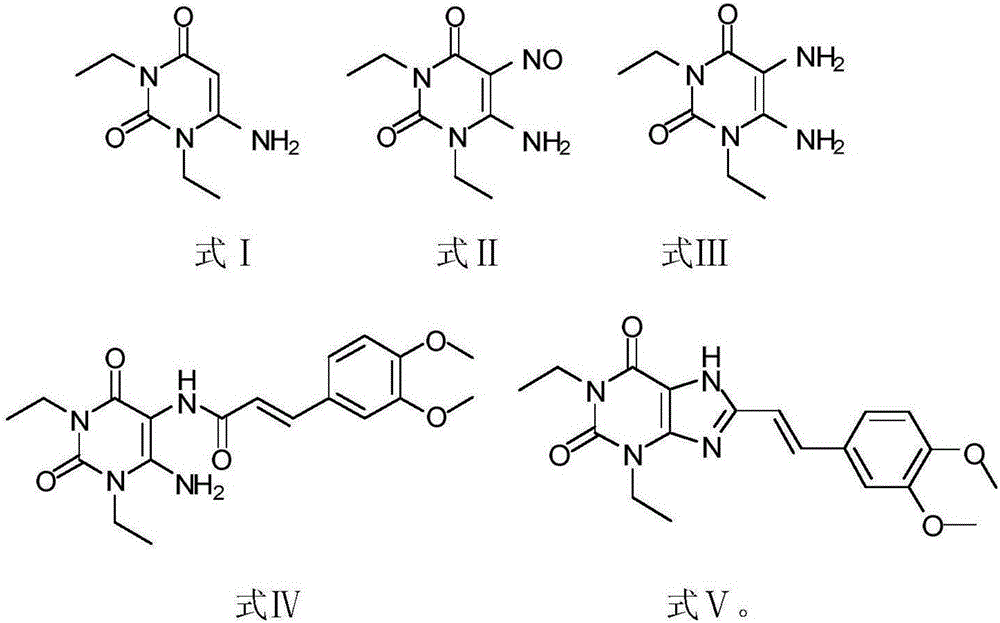
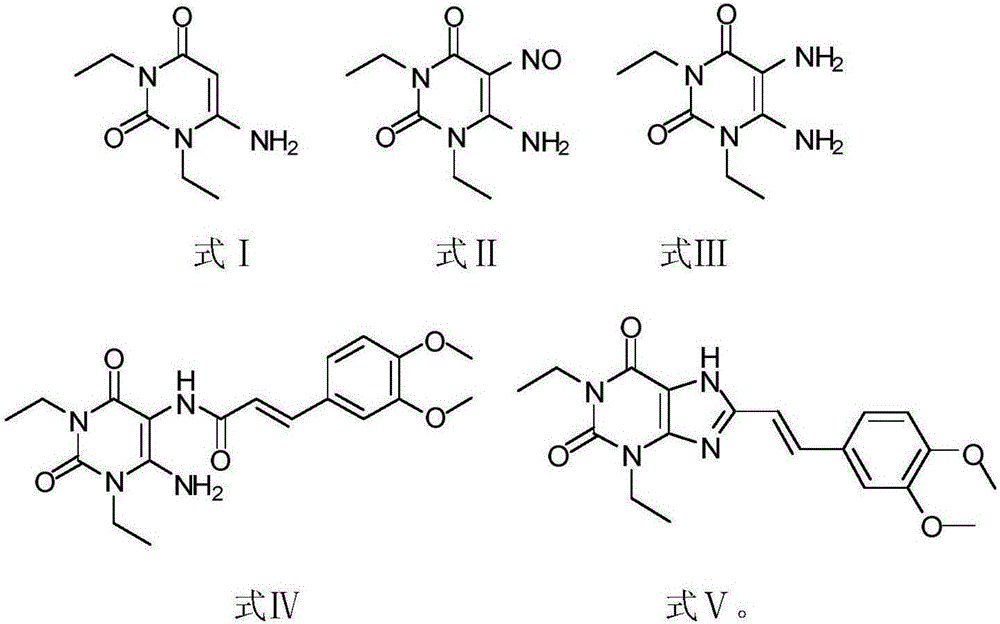
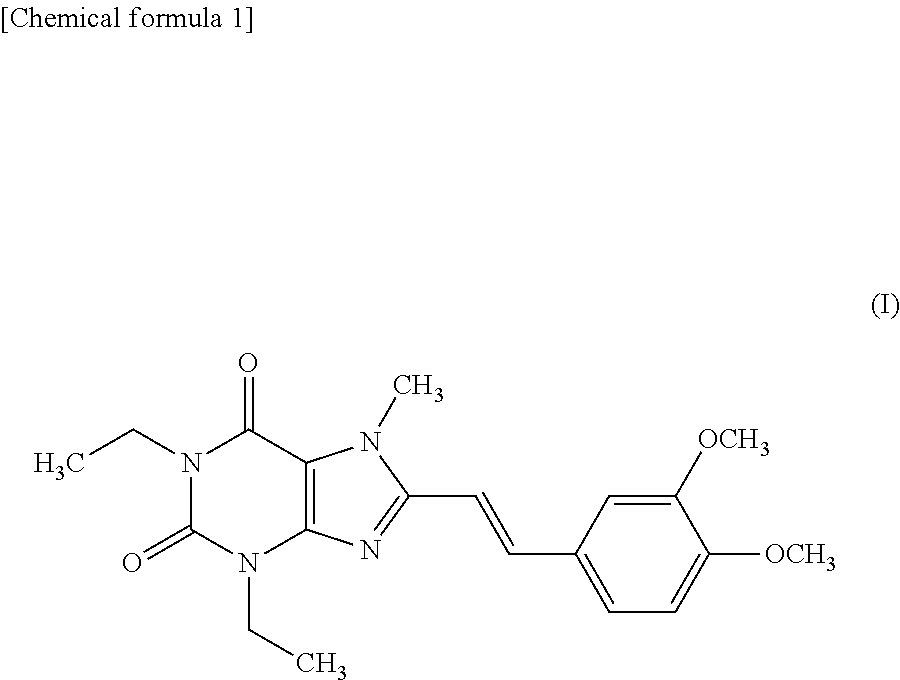
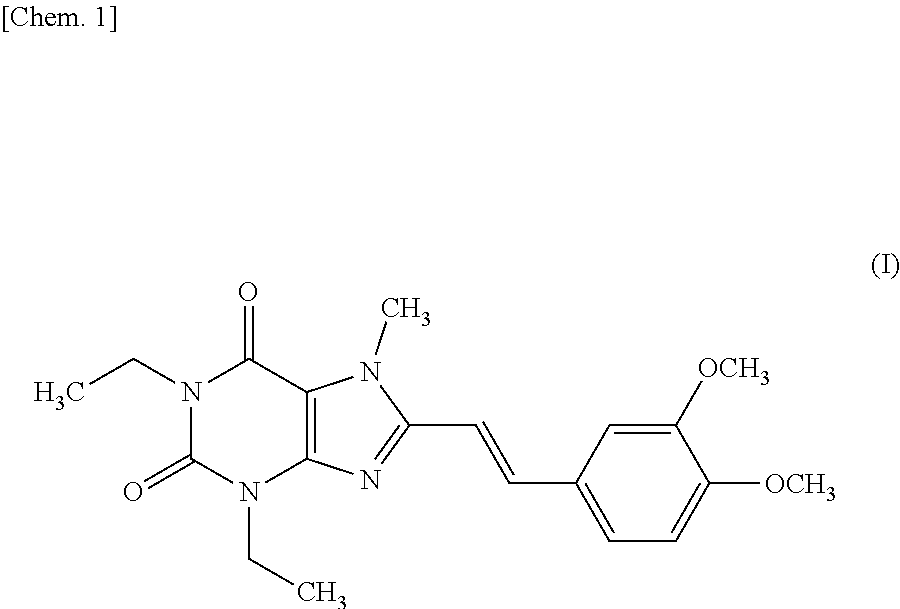
The invention relates to a preparation method for Istradefylline represented by a formula (I) shown in the description. The method comprises the following steps: subjecting 6-amino-1,3-diethyl-5-nitroso-1H-pyrimid-2,4-dione represented by a formula (II) shown in the description, which serves as a raw material, to catalytic reduction and salt forming, so as to obtain 5,6-diamino-1,3-diethyl-1H-pyrimid-2,4-dione hydrochloride represented by a formula (III) shown in the description; then, carrying out acetylation on the compound (III), so as to obtain N-(6-amino-1,3-diethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimid-5-yl)acetamide represented by a formula (IV) shown in the description; carrying out further ring closing, so as to obtain 1,3-diethyl-8-methyl-1H-purin-2,6(3H,7H)-dione represented by a formula (V) shown in the description; and carrying out methylation on the compound (V) firstly, and then, subjecting the methylation product to a condensation reaction with veratraldehyde represented by a formula (VII) shown in the description, thereby obtaining the end product Istradefylline. According to the novel preparation method for the Istradefylline, provided by the invention, raw materials, which are readily available industrially and are low in price, are used, the production process is more environmentally friendly, and the obtained product Istradefylline is high in yield and purity, thereby having a relatively high practical value.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
Istradefylline raw material drug and preparation method thereof
ActiveCN108864096ASimple processProcess stabilityNervous disorderOrganic chemistryPharmacyIstradefylline
The invention relates to an istradefylline raw material drug and a preparation method thereof. Specifically, the invention relates to an istradefylline raw material drug. A compound shown in formula III is not higher than 0.5% and is a medicine preparation consisting of the istradefylline raw material drug and a pharmacologically acceptable carrier and / or diluent. The raw material drug and the preparation thereof have better safety, effectiveness and stability. The formula III is shown in the description.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Therapeutic and/or prophylactic agent for lewy body disease
The present invention provides a therapeutic and / or prophylactic agent and the like, comprising istradefylline as an active ingredient, for Lewy body disease (for example, Parkinson's disease dementia, diffuse Lewy body disease, dementia with Lewy bodies, movement disorder associated with Lewy body disease and the like).
Owner:KYOWA HAKKO KIRIN CO LTD
Pharmaceutical composition containing istradefylline and application thereof
ActiveCN104814963ALittle side effectsLow cost of treatmentNervous disorderHeterocyclic compound active ingredientsDiseaseSide effect
The invention relates to a pharmaceutical composition and application thereof, specifically to a pharmaceutical composition containing istradefylline and application thereof, belonging to the field of medicine. To overcome disadvantages of conventional neurodegenerative disease therapeutics in neurodegenerative disease therapeutics, especially the defects of great side effect and high treatment cost of therapeutics for the Parkinson disease, the invention provides the pharmaceutical composition containing istradefylline. When the pharmaceutical composition containing istradefylline is applied to treatment of the Parkinson disease, side-effects of drugs in treatment of the Parkinson disease are substantially reduced, treatment cost for the Parkinson disease is lowered down at the same time, and a breakthrough is made to the golden rule that levodopa has to be used in clinical treatment of the Parkinson disease; thus, the pharmaceutical composition containing istradefylline has good medical application prospects.
Owner:NANJING ANGGU PHARMA TECH
Preparation method for medicine-istradefylline crystal form II for treating Parkinson
The invention discloses a preparation method for a medicine-istradefylline crystal form II for treating Parkinson. After the istradefylline and mixed solvent are mixed, mixture is heated to 70-90 DEGC to be dissolved, then, the mixture is quickly cooled to 0-5 DEG C, and crystallization is carried out to obtain the istradefylline crystal form II for treating Parkinson. The stability of the istradefylline crystal form II for treating Parkinson is equivalent to the stability of the traditional istradefylline crystal form II, yield and purity are higher, the quality of a product is greatly improved, in addition, the bioavailability of the istradefylline crystal form II can be improved so as to be favorable for the medicine processing and user in a medicine composite, and meanwhile, qualitative and quantitative information can be provided so as to have an important meaning for further researching the curative effect of the solid medicine.
Owner:董丹丹
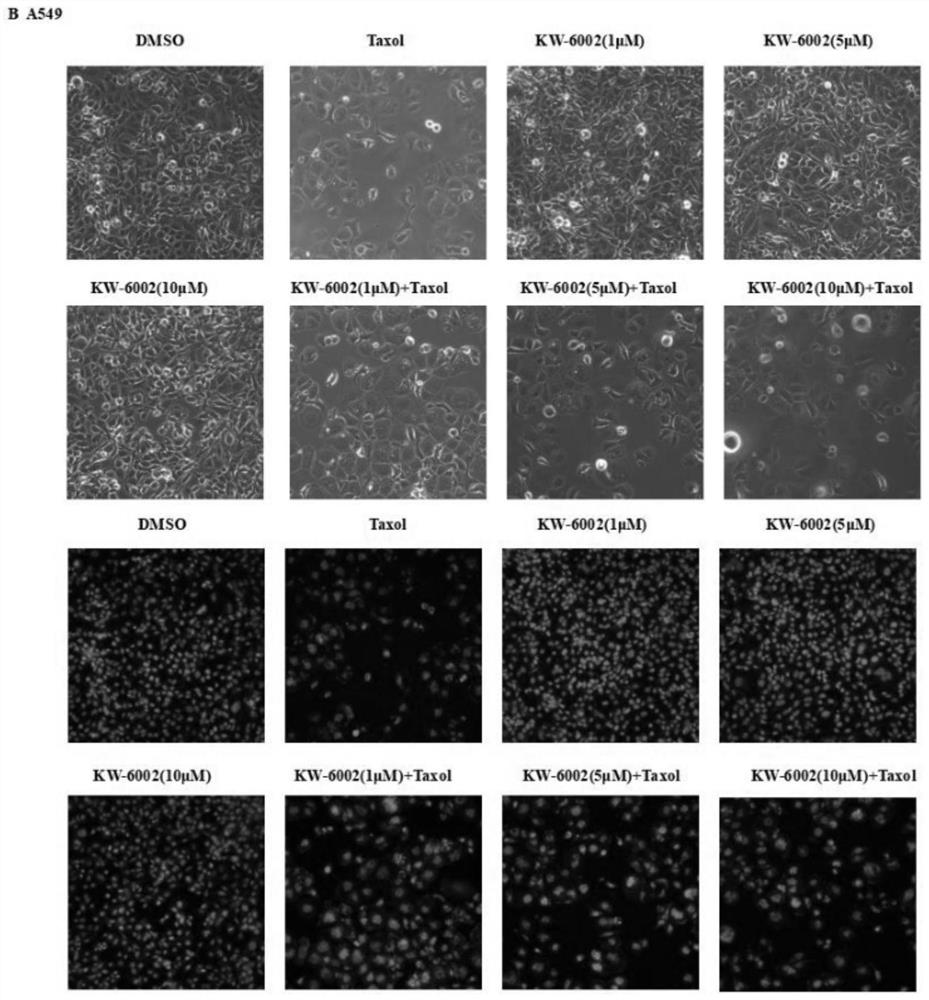
Pharmaceutical application of compound istradefylline for reversing drug resistance of paclitaxel
PendingCN113230259APromote growthPromote apoptosisAntineoplastic agentsHeterocyclic compound active ingredientsTolerabilityTherapeutic effect
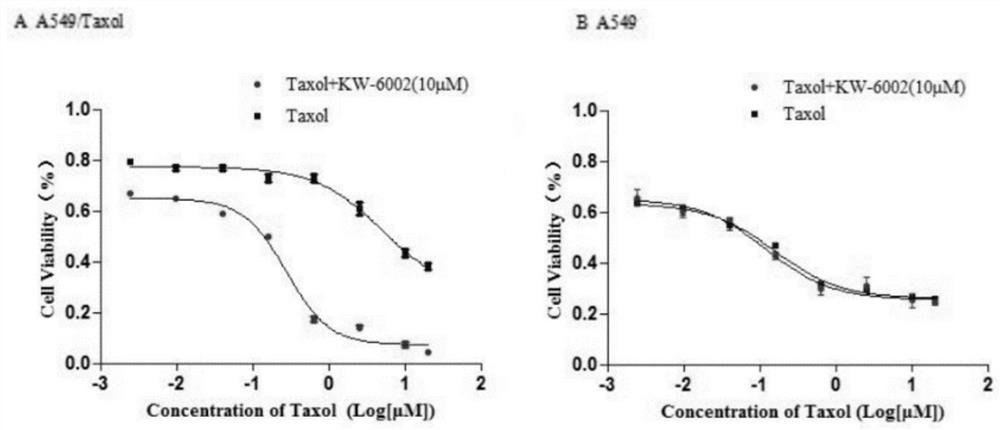
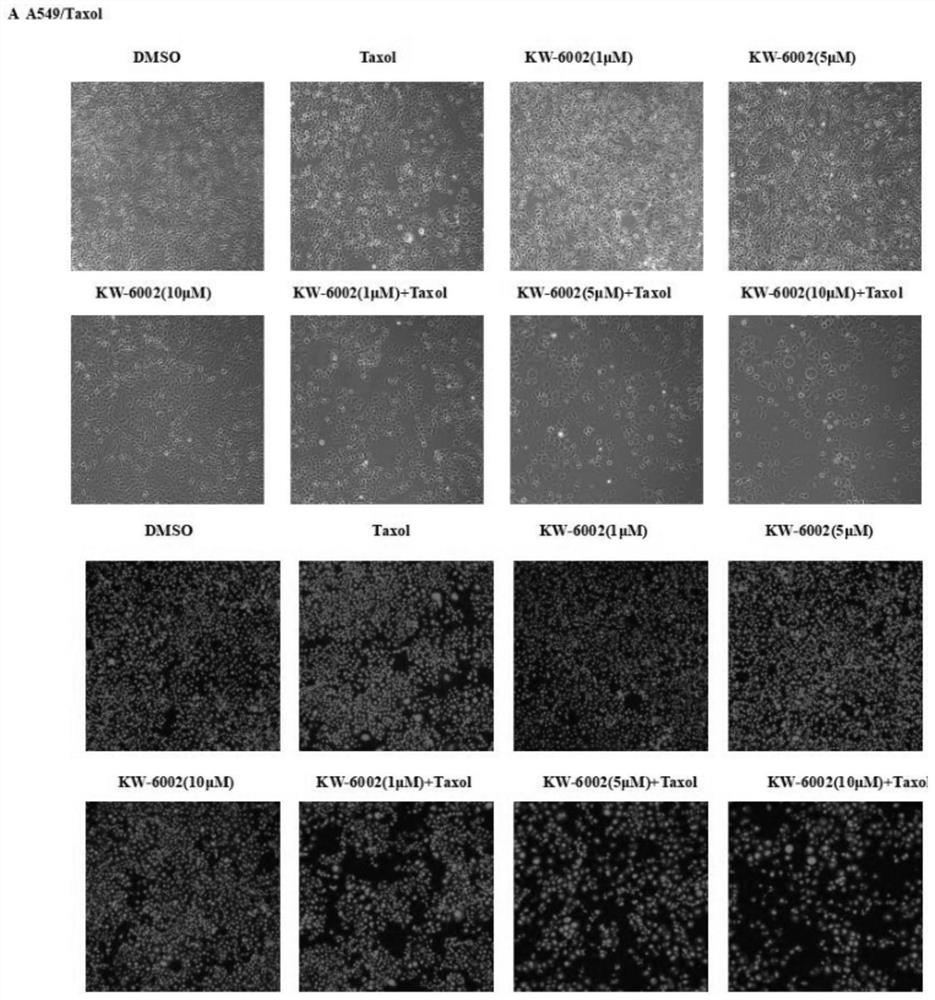
The invention discloses an application of istradefylline in preparation of drugs for reversing drug resistance of tumor cells, and relates to the technical field of treatment of drug resistance of tumor cells. The istradefylline and the paclitaxel are combined to be used for preparing the medicine for reversing the drug resistance of the tumor paclitaxel. The researches find that the istradefylline can significantly reduce the expression level of MDR1 genes and proteins, and the combination of the istradefylline and the chemotherapeutic drug paclitaxel can significantly improve the sensitivity of drug-resistant cells to the chemotherapeutic drug, so that the tumor drug resistance is reversed, and the curative effect is greatly improved. Animal experiments also prove that istradefylline can effectively inhibit the growth speed of subcutaneous tumors of a drug-resistant animal model and reverse the drug resistance of paclitaxel. The results show that the istradefylline is combined with the paclitaxel, so that the treatment effect of the paclitaxel on tolerant tumors can be effectively enhanced. The condition that many malignant tumor cells have drug resistance to paclitaxel is clinically found, and therefore istradefylline has good development value and application prospects.
Owner:WENZHOU MEDICAL UNIV
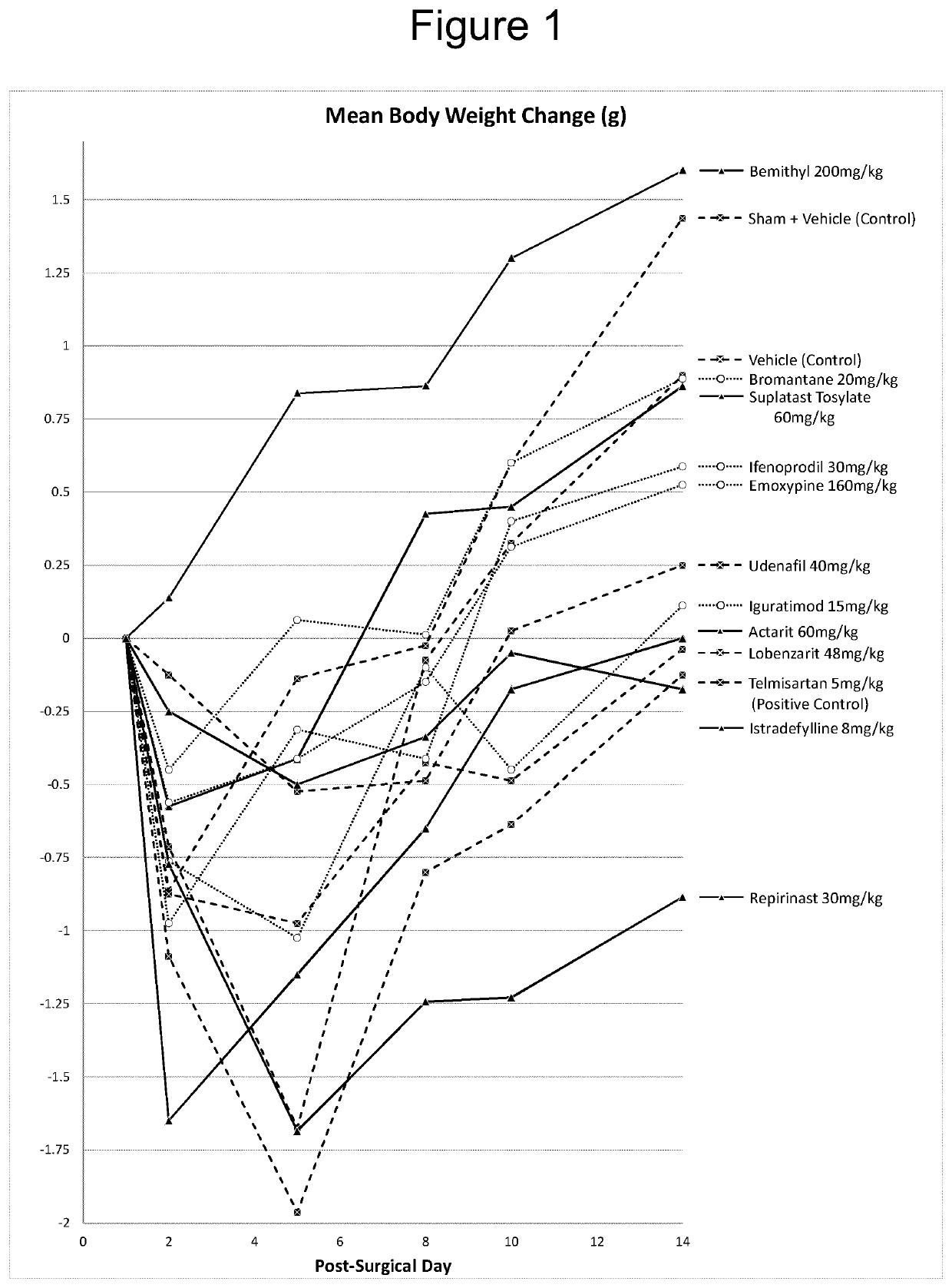
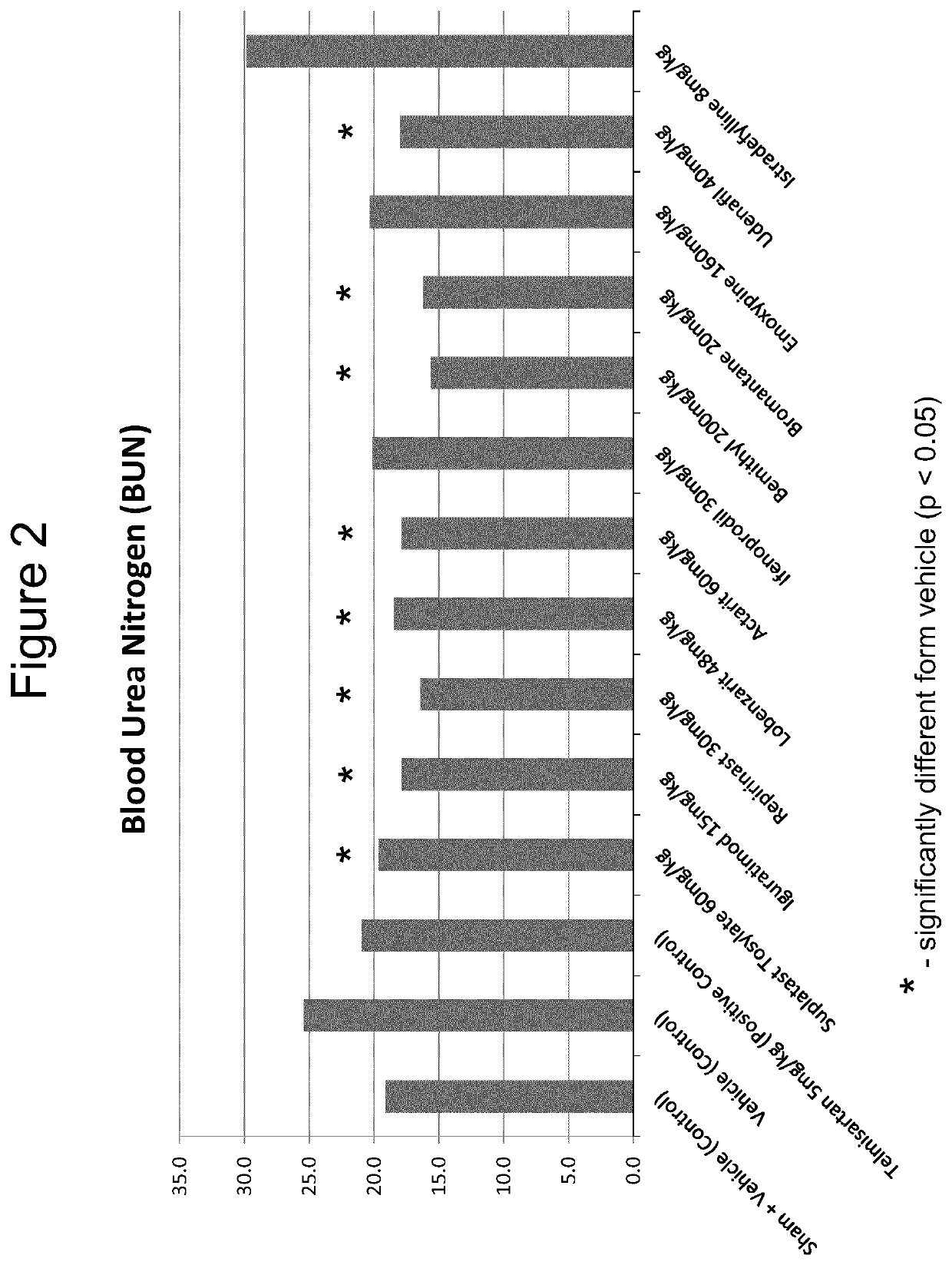
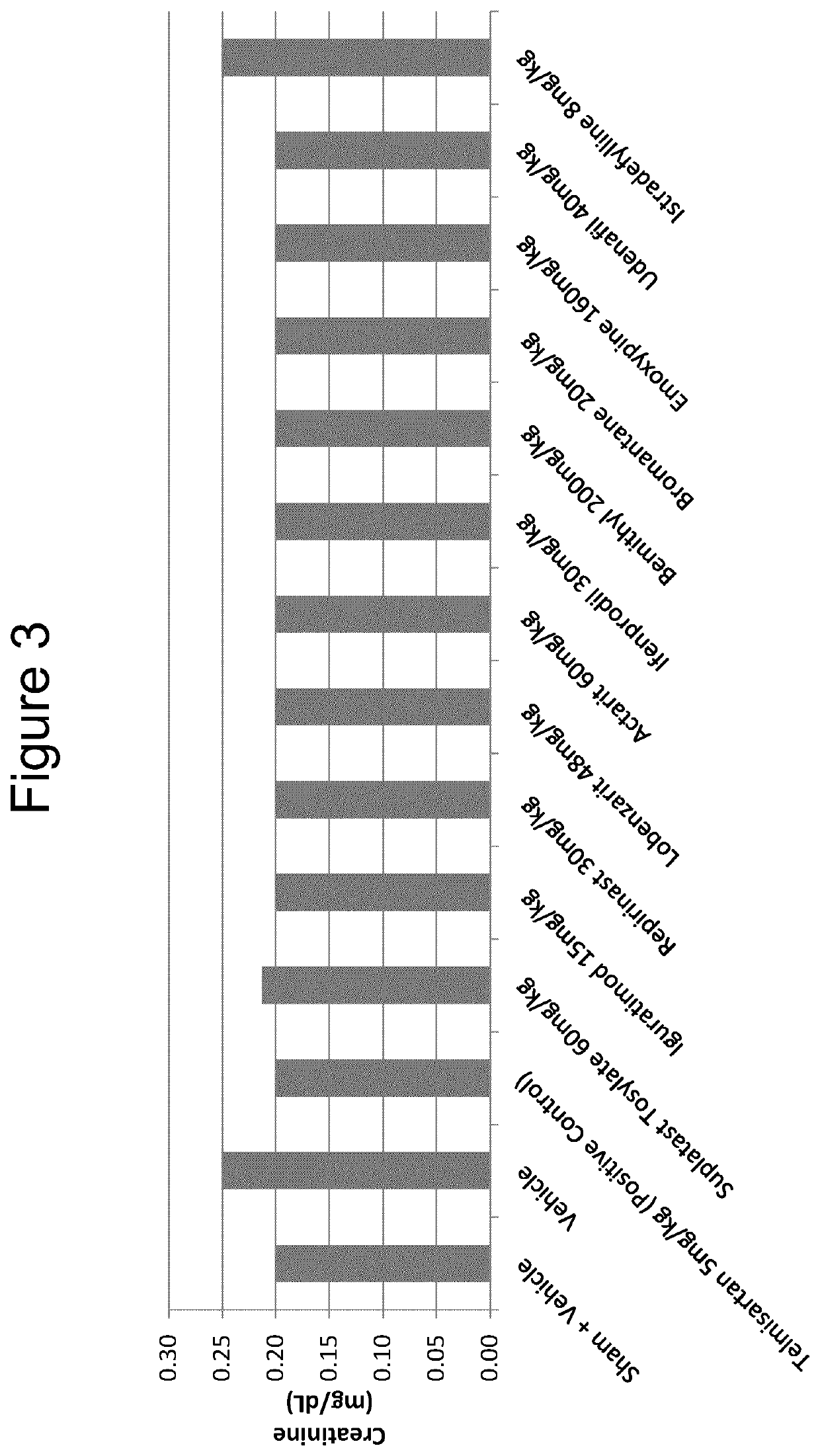
The use of actarit in the prophylaxis or treatment of renal fibrosis or kidney disease
PendingUS20210260000A1Reduction in renal fibrosisPeptide/protein ingredientsUrinary disorderEmoxypineRepirinast
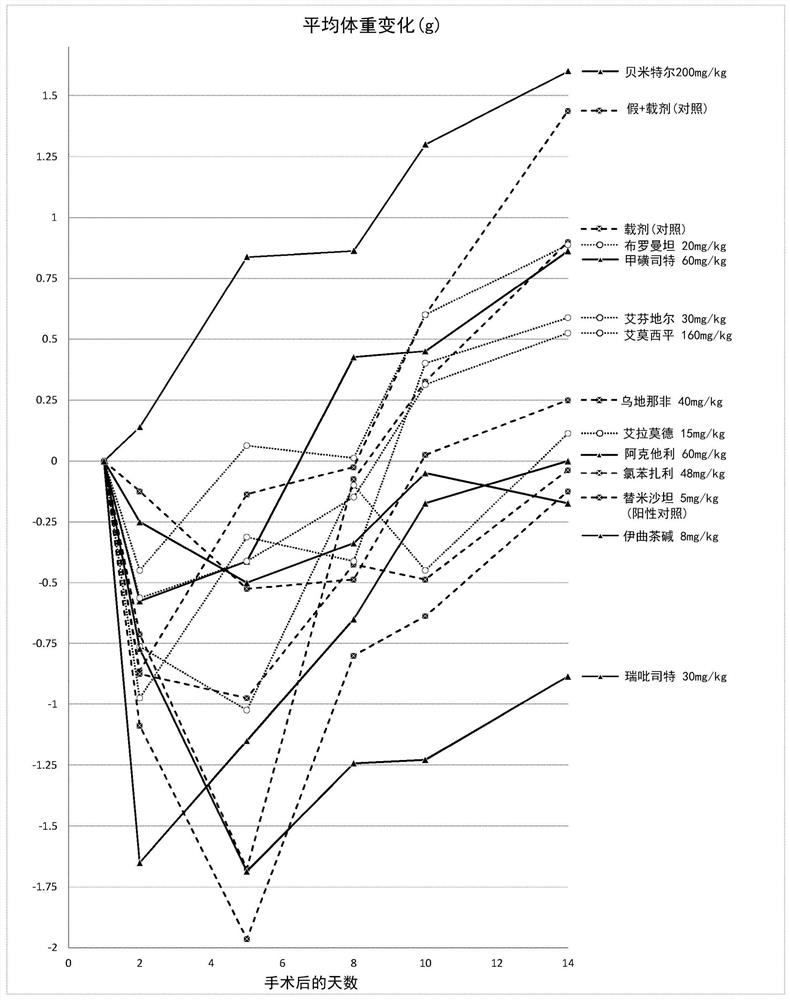
Iguratimod, Repirinast, Lobenzarit, Actarit, Ifenprodil, Bemithyl, Bromantane, Emoxypine, Udenafil, and / or Istradefylline are used for the treatment or prophylaxis of renal fibrosis, kidney disease, or chronic kidney disease in a subject.
Owner:ALGERNON PHARMA INC
Istradefylline containing pharmaceutical composition
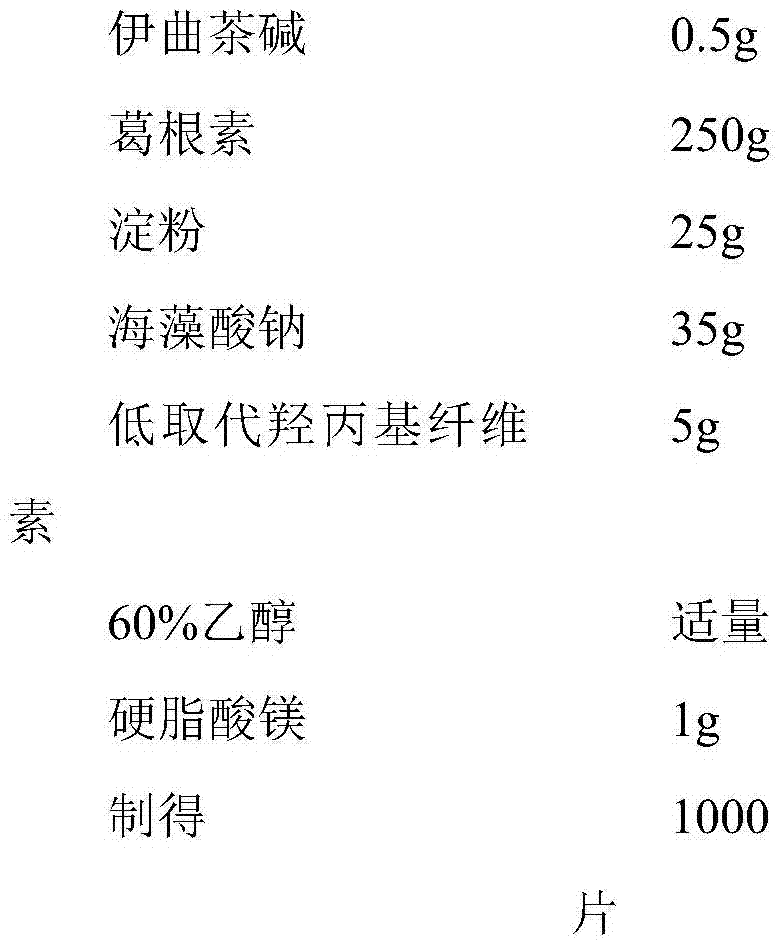
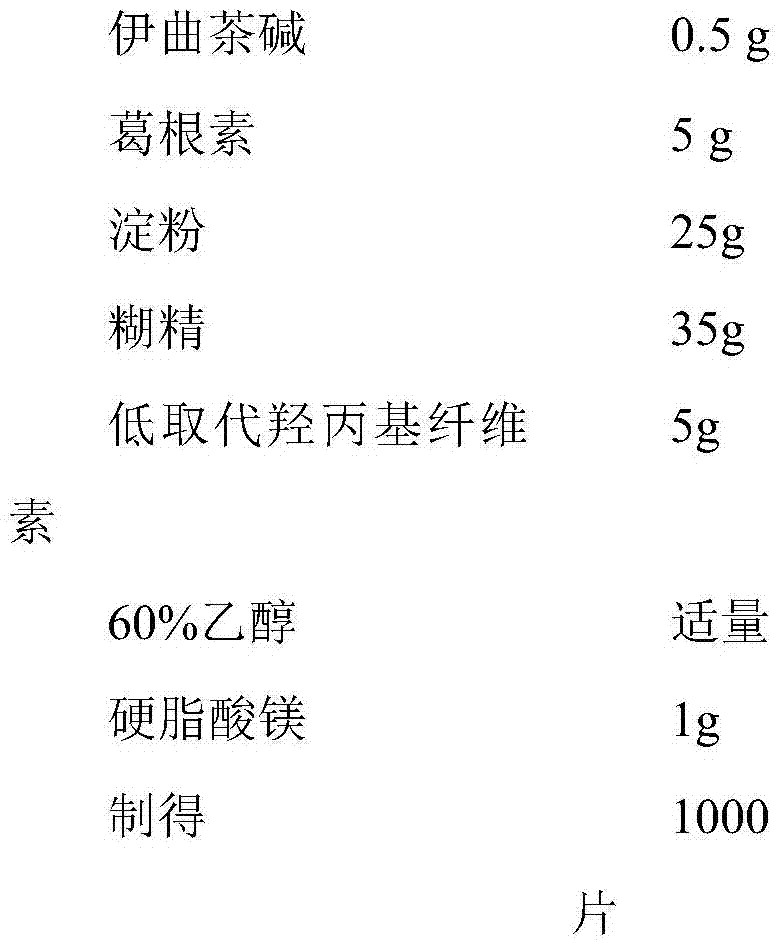
InactiveCN106214652ANervous disorderPharmaceutical non-active ingredientsAntioxidantLow-substituted hydroxypropylcellulose
The invention discloses an istradefylline containing pharmaceutical composition. The composition is prepared from, by weight, 20-50 parts of istradefylline, 10-20 parts of puerarin, 5-12 parts of starch, 1-9 parts of low-substituted hydroxypropyl cellulose, 2-6 parts of magnesium stearate, 2-8 parts of antioxidant, 3-5 parts of mannitol, 2-10 parts of superfine silica powder, 2-10 parts of chitosan, 2-8 parts of sodium alginate and 3-10 parts of lactose. A preparation process of the composition includes the steps of weighing and evenly mixing istradefylline, puerarin, starch, low-substituted hydroxypropyl cellulose, magnesium stearate, antioxidant, mannitol, superfine silica powder, chitosan, sodium alginate and lactose according to the amounts in the formula, additionally adding a proper amount of 60% ethyl alcohol to mixed powder to be evenly mixed to prepare a soft material, conducting granulating through a 16-mesh screen, conducting drying at 60 DEG C or below, conducting size stabilizing after drying is completed, evenly mixing granules with dry particles, and conducting tabletting to obtain the composition. The composition has the advantage of treating the Parkinson's disease.
Owner:合肥美利康医药技术股份有限公司
Therapeutic agent for parkinson's disease
A therapeutic agent for Parkinson's disease containing istradefylline as an effective ingredient being characterized in exhibiting more expression of a shortening effect of the OFF time by administration to a patient of Parkinson's disease of 65 or more years old as compared with administration to a patient of Parkinson's disease of younger than 65 years old.
Owner:KYOWA HAKKO KIRIN CO LTD
Preparation method for removing methyl impurities from istradefylline
ActiveCN113248505AImprove quality controlHigh purityOrganic chemistryIstradefyllinePhysical chemistry
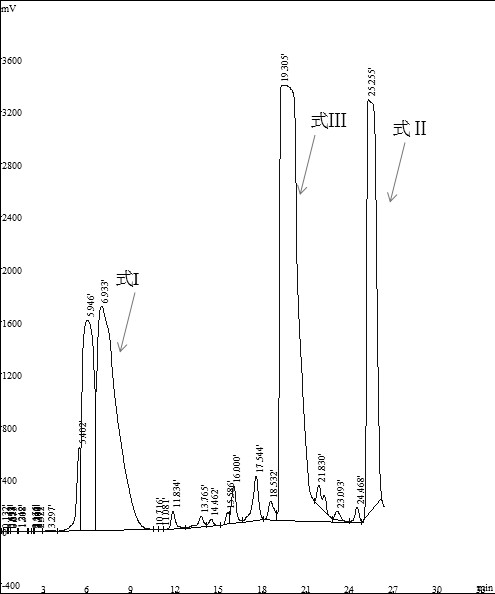
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a preparation and purification method for removing methyl impurities from istradefylline. Istradefylline is used as a raw material to prepare three high-purity demethylated impurity compounds I, II and III, the method is simple in process, easy to operate, simple in purification and high in yield, the prepared impurities are high in purity, and qualified impurity reference substances can be provided for quality control of istradefylline. The istradefylline impurity reference substance prepared by the invention can provide an important reference basis for monitoring the impurity in the research and development of an istradefylline process, improves the quality monitoring level of istradefylline, and has great significance and practical value for the development of medicinal istradefylline.
Owner:江苏华阳制药有限公司
A kind of synthetic method of istradefylline
The invention discloses a synthetic method of istradefylline, and belongs to the technical field of medicines. The synthesis method of the istradefylline mainly comprises the steps of acylation, ringclosing, methylation and the like. The method is high in yield, simple in step and single in solvent, wherein reaction selectivity can be effectively promoted and side reactions are reduced; after ring closing is finished, dimethyl sulfate is dropwise added for a methylation reaction by means of the alkalinity of dimethyl sulfate. The method can be more thorough and cleaner than a dimethyl carbonate reaction in the prior art. The whole process can be carried out in one reaction kettle, wherein the solvent only relates to ethanol and subsequently refined methanol and can be recycled, so that the synthesis method has advantages of high reaction selectivity and yield.
Owner:LIANYUNGANG GUIKE PHARMA
Preparation method of istradefylline intermediate
The invention provides a preparation method of an istradefylline intermediate, namely (E)-1,3-diethyl-6-amino-5-(3,4-dimethoxyphenylacryloyl)aminoauracil. The preparation method is realized through the following steps: 1,3-diethyl-5,6-diaminouracil serves as a raw material, dichloromethane serves as a solvent, triethylamine serves as an acid-binding agent, (E)3,4-dimethoxyphenylacryloyl chloride is dropwise added slowly, reacting and acid washing are conducted, water is added for crystallization, and thus the (E)-1,3-diethyl-6-amino-5-(3,4-dimethoxyphenylacryloyl)aminoauracil is obtained. According to the preparation method, a product is promoted to precipitate from an organic layer, a reactant and a by-product can be separated effectively, the obtained istradefylline intermediate is highin purity, and operation is easy and convenient.
Owner:SHANDONG XINHUA PHARMA CO LTD
A kind of istradefylline crude drug and preparation method thereof
ActiveCN108864096BSimple processProcess stabilityNervous disorderOrganic chemistryIstradefyllineDrugs preparations
The invention relates to an istradefylline raw material drug and a preparation method thereof. Specifically, the invention relates to an istradefylline raw material drug. A compound shown in formula III is not higher than 0.5% and is a medicine preparation consisting of the istradefylline raw material drug and a pharmacologically acceptable carrier and / or diluent. The raw material drug and the preparation thereof have better safety, effectiveness and stability. The formula III is shown in the description.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
A kind of preparation method of istradefylline
The invention relates to a preparation method for Istradefylline represented by a formula (I) shown in the description. The method comprises the following steps: subjecting 6-amino-1,3-diethyl-5-nitroso-1H-pyrimid-2,4-dione represented by a formula (II) shown in the description, which serves as a raw material, to catalytic reduction and salt forming, so as to obtain 5,6-diamino-1,3-diethyl-1H-pyrimid-2,4-dione hydrochloride represented by a formula (III) shown in the description; then, carrying out acetylation on the compound (III), so as to obtain N-(6-amino-1,3-diethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimid-5-yl)acetamide represented by a formula (IV) shown in the description; carrying out further ring closing, so as to obtain 1,3-diethyl-8-methyl-1H-purin-2,6(3H,7H)-dione represented by a formula (V) shown in the description; and carrying out methylation on the compound (V) firstly, and then, subjecting the methylation product to a condensation reaction with veratraldehyde represented by a formula (VII) shown in the description, thereby obtaining the end product Istradefylline. According to the novel preparation method for the Istradefylline, provided by the invention, raw materials, which are readily available industrially and are low in price, are used, the production process is more environmentally friendly, and the obtained product Istradefylline is high in yield and purity, thereby having a relatively high practical value.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
A kind of preparation method of istradefylline impurity standard substance
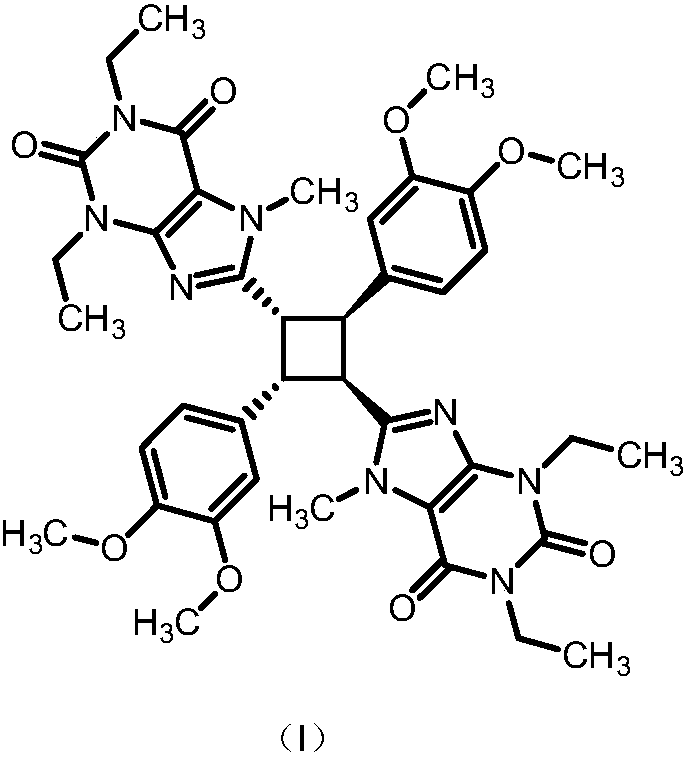
ActiveCN106478673BEquipment condition is not highEasy to implementOrganic chemistryIstradefyllinePurine
The invention discloses a preparation method of an istradefylline impurity standard. Istradefylline is subjected as a raw material to Lewis acid catalysis [2+2] cycloaddition, the reaction product is then refined to obtain pure 8,8'-((1R,2R,3S,4S)-2,4-bis(3,4-dimethoxyphenyl)cyclobutane-1,3-diyl)bis(1,3-diethyl-7-methyl-1H-purine-2,6(3H,7H)-dione), and the content of the pure product is calibrated by conventional analytic means. The preparation method provided herein is simple and has a short preparation cycle, and the calibrated content is higher than 99.0%. Istradefylline impurity provided herein may act as an impurity standard, applied to the qualitative and quantitative study and detection on istradefylline material and its preparation impurities.
Owner:HEFEI JIUNUO MEDICAL TECH
Synthetic method of istradefylline
ActiveCN111548351AReduce hydrolysisLess side effectsOrganic chemistryIstradefyllineCombinatorial chemistry
The invention discloses a synthetic method of istradefylline, and belongs to the technical field of medicines. The synthesis method of the istradefylline mainly comprises the steps of acylation, ringclosing, methylation and the like. The method is high in yield, simple in step and single in solvent, wherein reaction selectivity can be effectively promoted and side reactions are reduced; after ring closing is finished, dimethyl sulfate is dropwise added for a methylation reaction by means of the alkalinity of dimethyl sulfate. The method can be more thorough and cleaner than a dimethyl carbonate reaction in the prior art. The whole process can be carried out in one reaction kettle, wherein the solvent only relates to ethanol and subsequently refined methanol and can be recycled, so that the synthesis method has advantages of high reaction selectivity and yield.
Owner:LIANYUNGANG GUIKE PHARMA
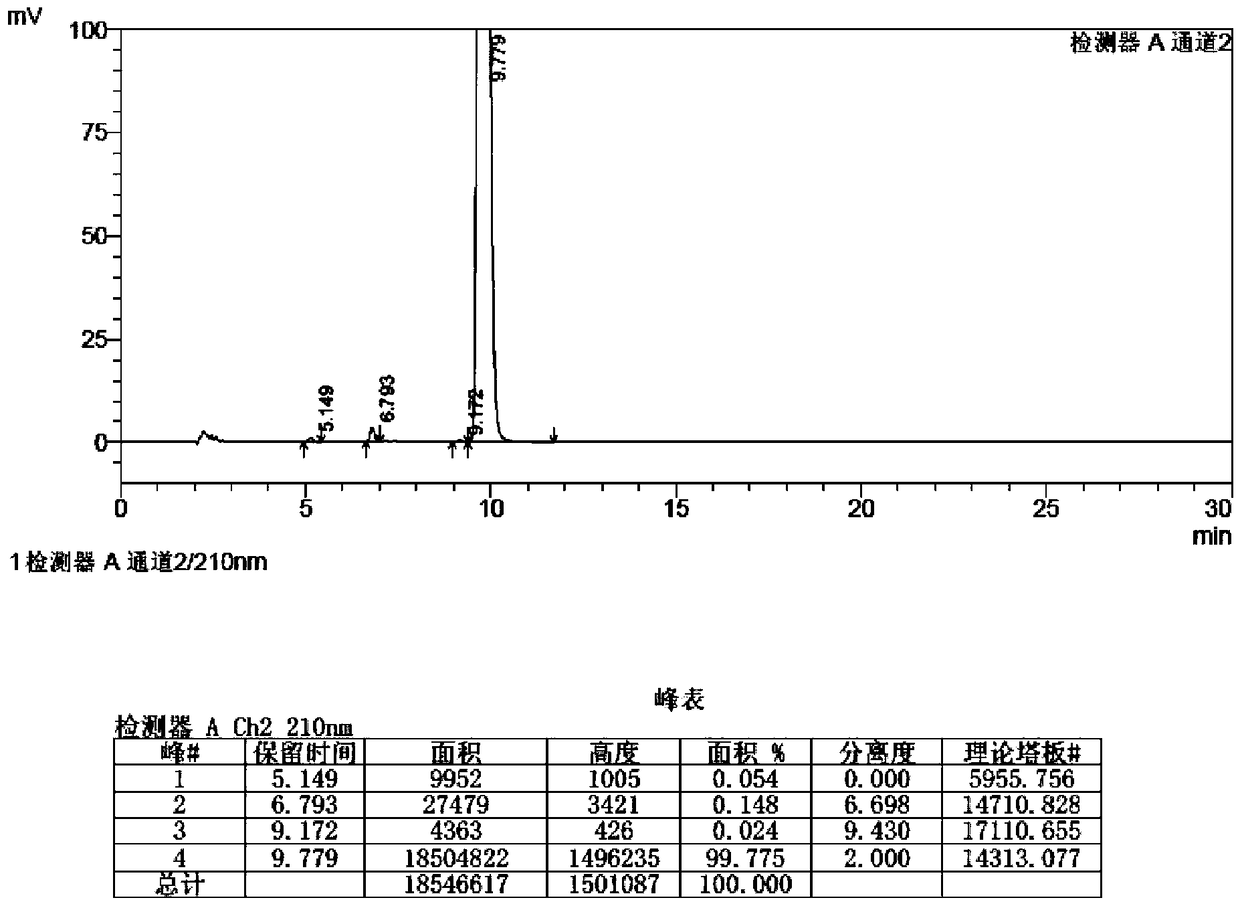
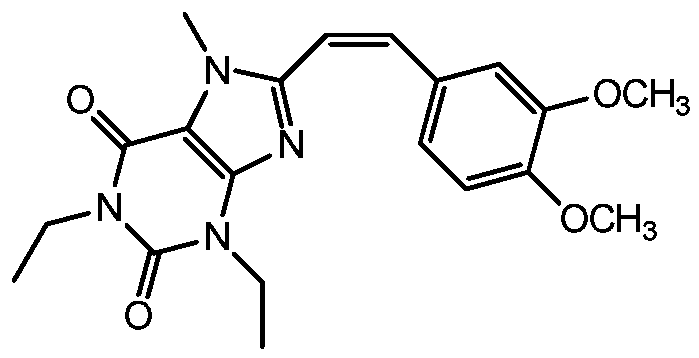
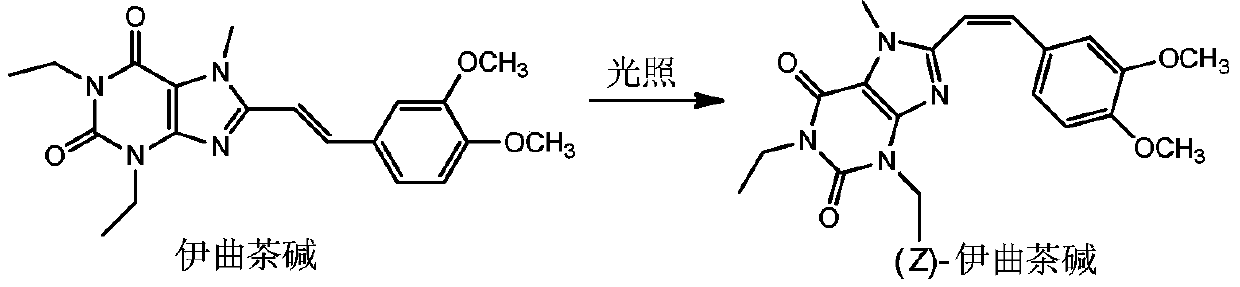
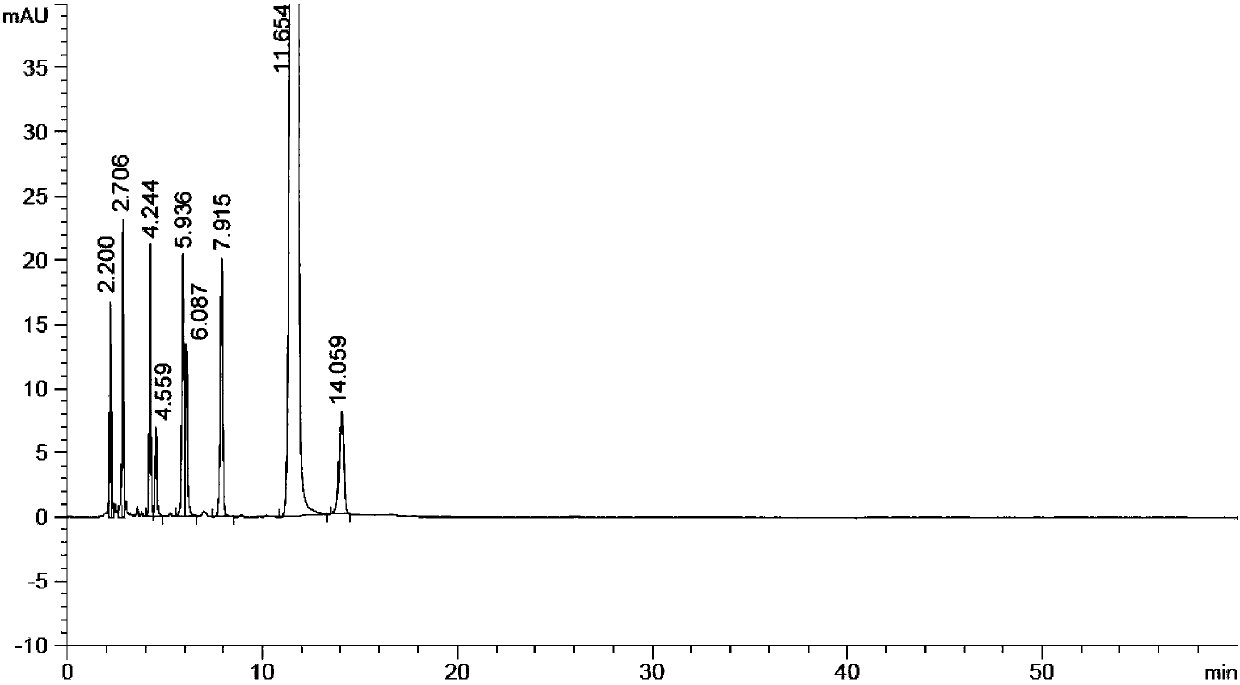
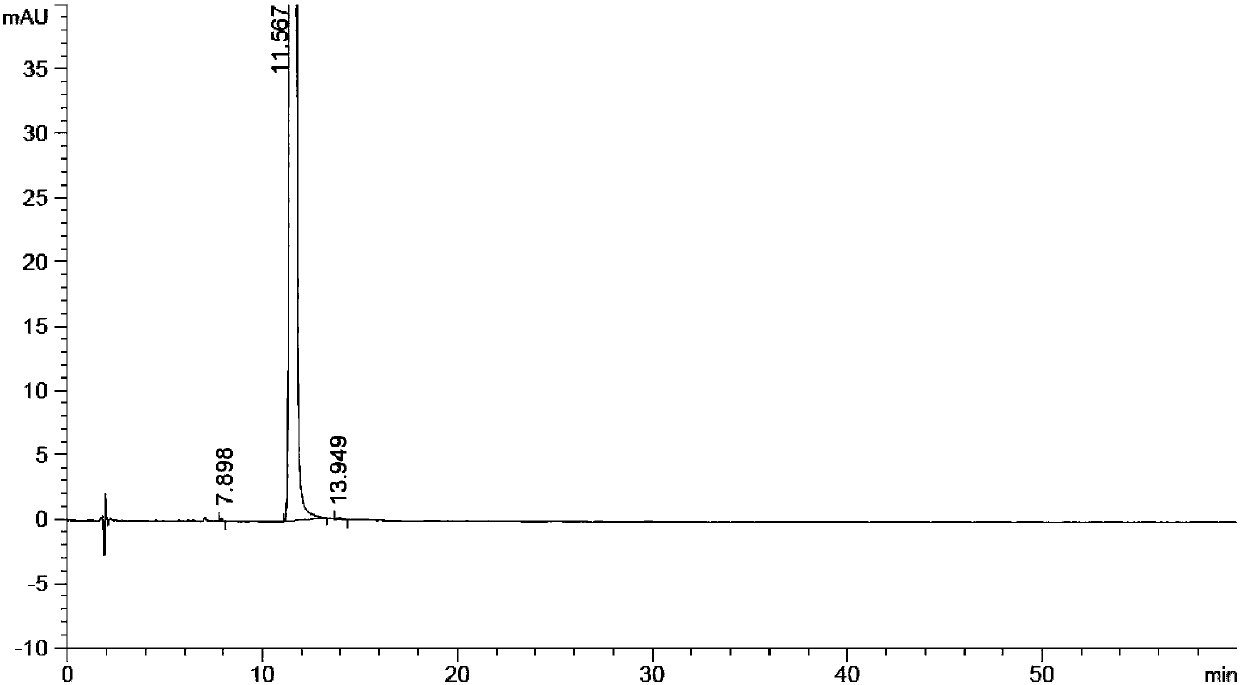
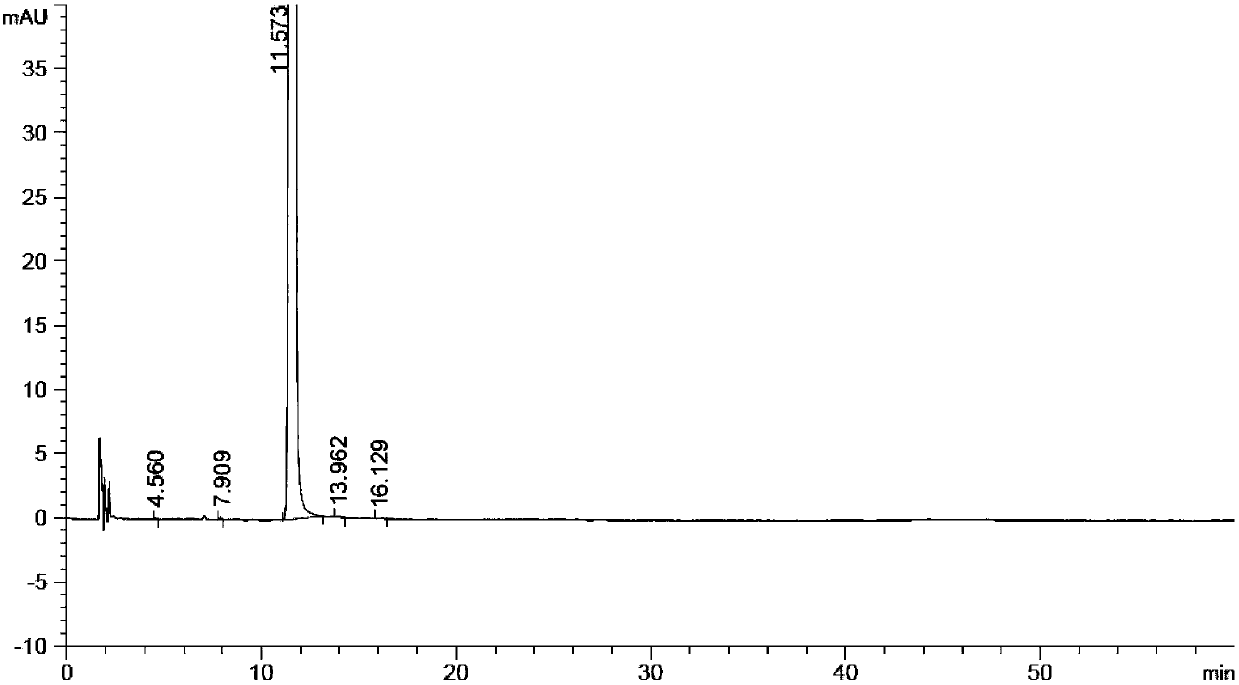
Method for preparing (Z)-istradefylline
The invention provides a method for preparing (Z)-istradefylline, wherein acetonitrile-water is used as a solvent, separation preparation is performed through a reverse high performance liquid chromatography preparation method after light irradiation, and after acetonitrile is distilled off, post-treatment is performed by using a freeze-drying technology. According to the present invention, the post-treatment is simple, and high temperature is not required, such that the generation of side reactions and impurities is avoided, and the prepared target has high purity; the yield is high, and reaches more than 60%; and the method has characteristics of controllable process and easy operation.
Owner:SHANDONG XINHUA PHARMA CO LTD
A kind of high performance liquid chromatography analysis method of istradefylline related substances
The invention discloses a high-performance liquid chromatography analytical method for istradefylline related substances. A reversed phase chromatographic column and an ultraviolet detector are adopted for isocratic elution with acetonitrile-phosphate buffer as a mobile phase. All known impurities in raw materials and preparations of istradefylline can be analyzed simultaneously, contents of the known impurities can be effectively controlled by a main component self contrast method with correction factors, a degree of separation between each impurity peak and a main peak is larger than 1.5, and purity of each impurity peak and the main peak is 1.0. The high-performance liquid chromatography analytical method for the istradefylline related substances is a high-specificity simple analytical method for quality control analysis of the raw materials and the preparations of the istradefylline.
Owner:HEFEI JIUNUO MEDICAL TECH
A pharmaceutical composition containing istradefylline and its application
ActiveCN104814963BGood treatment effectNeuroprotectiveNervous disorderHeterocyclic compound active ingredientsDiseaseSide effect
The invention relates to a pharmaceutical composition and application thereof, specifically to a pharmaceutical composition containing istradefylline and application thereof, belonging to the field of medicine. To overcome disadvantages of conventional neurodegenerative disease therapeutics in neurodegenerative disease therapeutics, especially the defects of great side effect and high treatment cost of therapeutics for the Parkinson disease, the invention provides the pharmaceutical composition containing istradefylline. When the pharmaceutical composition containing istradefylline is applied to treatment of the Parkinson disease, side-effects of drugs in treatment of the Parkinson disease are substantially reduced, treatment cost for the Parkinson disease is lowered down at the same time, and a breakthrough is made to the golden rule that levodopa has to be used in clinical treatment of the Parkinson disease; thus, the pharmaceutical composition containing istradefylline has good medical application prospects.
Owner:NANJING ANGGU PHARMA TECH
The use of actarit in the prophylaxis or treatment of renal fibrosis or kidney disease
Owner:ALGERNON PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com