Istradefylline synthesis process
A technology for istradefylline and a synthesis process, applied in the field of istradefylline synthesis technology, can solve the problems of complex process, obstacles to large-scale production of istradefylline, and high product cost, and achieves simple synthesis route, easy purification, The effect of the best stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
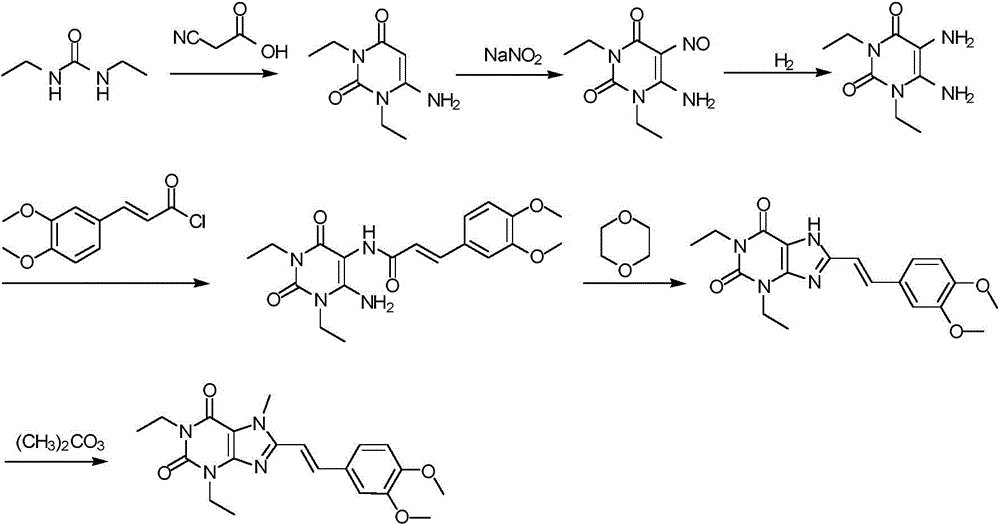
[0038] Preparation of istradefylline:
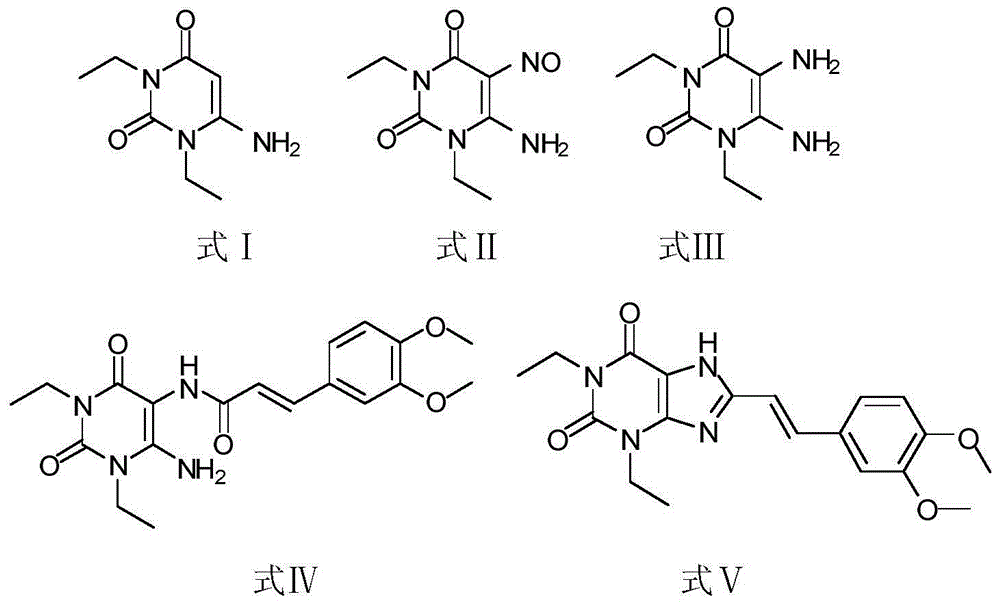
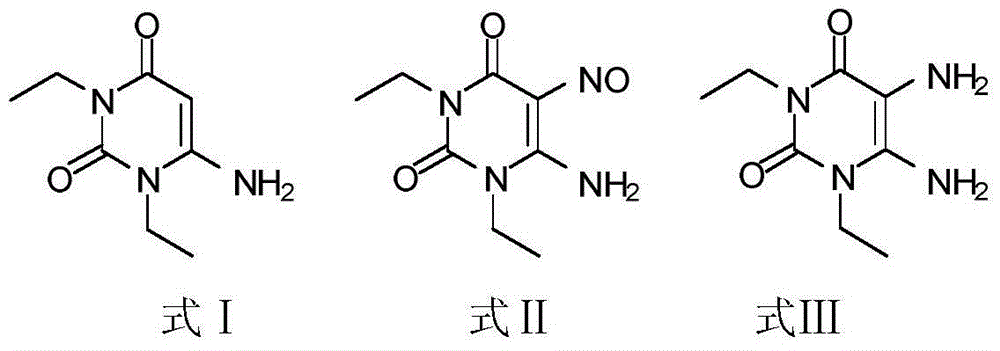
[0039] 1) Put 180g of 1,3-diethylformamide and 125g of cyanoacetic acid in 185ml of acetic anhydride for ring formation reaction. The reaction condition is to heat and reflux at 50°C for 2.5h. After the reaction is completed, add sodium hydroxide, adjust the pH to 7, and pump filtered, washed twice with water, and the resulting white solid was compound Ⅰ;
[0040] 2) Add 260 g of compound I to 1 L of acetic acid, stir for 30 minutes, and then add sodium nitrite for nitration reaction. The reaction time is 3 hours. After the reaction is completed, the solid obtained by suction filtration and drying is compound II;
[0041] 3) Pass hydrogen through 260 g of compound II and 2.2 L of methanol for reduction reaction. The reaction time is 20 h. After the reaction is completed, the yellow solid obtained by HPLC detection and spin-drying is compound III;
[0042] 4) Under dark and oxygen-free conditions, 150g of compound III, 200g of 3-(3,4-dim...
Embodiment 2
[0046] Preparation of istradefylline:
[0047] 1) Put 180g of 1,3-diethylformamide and 120g of cyanoacetic acid in 180ml of acetic anhydride for a ring-forming reaction. The reaction condition is to heat and reflux at 60°C for 3 hours. After the reaction is completed, add sodium hydroxide, adjust the pH to 7, and pump filtered, washed twice with water, and the resulting white solid was compound Ⅰ;
[0048] 2) Add 260 g of compound I to 1 L of acetic acid, stir for 30 minutes, and then add sodium nitrite for nitration reaction. The reaction time is 3 hours. After the reaction is completed, the solid obtained by suction filtration and drying is compound II;
[0049] 3) Pass hydrogen through 260 g of compound II and 2.5 L of methanol for reduction reaction. The reaction time is 24 hours. After the reaction is completed, the yellow solid obtained by HPLC detection and spin-drying is compound III;
[0050] 4) Under dark and oxygen-free conditions, 150g of compound III, 200g of 3-(...
Embodiment 3
[0054] Preparation of istradefylline:
[0055] 1) Put 180g of 1,3-diethylformamide and 122g of cyanoacetic acid in 182ml of acetic anhydride for ring formation reaction, the reaction condition is to heat and reflux at 70°C for 3.5h, add sodium hydroxide after the reaction is completed, and adjust the pH to 7 Suction filtration, washing twice with water, the obtained white solid is compound Ⅰ;
[0056] 2) Add 260 g of compound I to 1 L of acetic acid, stir for 30 minutes, and then add sodium nitrite for nitration reaction. The reaction time is 3 hours. After the reaction is completed, the solid obtained by suction filtration and drying is compound II;
[0057] 3) Pass hydrogen through 260 g of compound II and 2.6 L of methanol for reduction reaction. The reaction time is 26 hours. After the reaction is completed, the yellow solid obtained by HPLC detection and spin-drying is compound III;
[0058] 4) Under dark and anaerobic conditions, 150g of compound III, 200g of 3-(3,4-dim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com