A kind of istradefylline crude drug and preparation method thereof
A technology of istraphylline and a compound, applied in the field of istraphylline raw materials and preparation thereof, can solve the problems of not effectively reducing or avoiding the side effects of istraphylline and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0057] The following examples are used to further describe the present invention, but these examples do not limit the scope of the present invention.
[0058] The experimental methods that do not specify specific conditions in the embodiments of the present invention usually follow conventional conditions or the conditions suggested by the raw material or commodity manufacturers. Reagents without specific sources are conventional reagents purchased on the market.
Embodiment 1
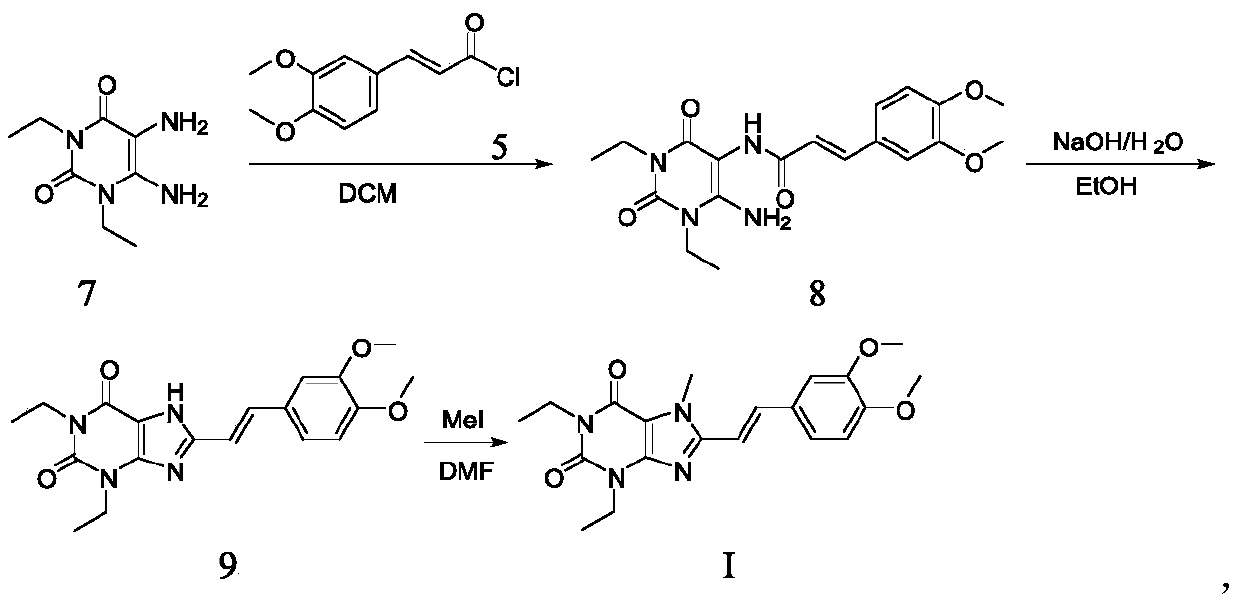
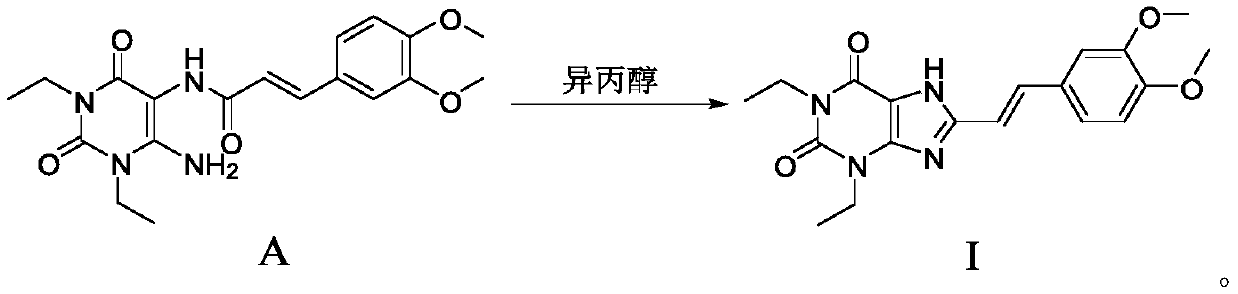
[0059] Example 1, EN-(6-amino-1,3-diethyl-2,4-dione-1,2,3,4-tetrahydropyrimidin-5-yl)-3-(3,4- Preparation of dimethoxy) propionamide (compound A)
[0060]
[0061] Suspend the compound of formula 1, 1,3-diethyl-5,6-diaminouracil (1.0kg, prepared according to EP0590919A1) in dichloromethane, add pyridine (1.6kg) under stirring, and dissolve in dichloromethane Compound 2 (1.54kg) in methane (3.5L) was added dropwise to the reaction system, and the addition was completed in about 1.5 hours. After stirring at room temperature for 15-20 hours, a saturated aqueous sodium bicarbonate solution was added dropwise to the reaction solution to adjust the pH to Around 7-8, a solid precipitated. After filtration, the filter cake was washed twice with water, and the filter cake was dried to obtain a pale yellow powdery solid product (1.56 kg) with a yield of 79.5%.
Embodiment 2
[0062] Example 2. Preparation of 8-((E)-2-(3,4-dimethoxyphenyl)vinyl)-1,3-diethyl-3,7-dihydro-1H-purine-2 Preparation of ,6-diketone (compound of formula I)
[0063]
[0064] Method A:
[0065] Add the compound of formula A (500g, 1.29mol) into a 10L reaction kettle, then add isopropanol (2.5L), stir, dissolve the solid sodium hydroxide (486g, 12.15mol) in water (2.0L), and then stir The reaction system was quickly added dropwise to the reaction system in about 30 minutes. The reaction was heated to 75-80°C. After 8-9 hours of reaction, TLC monitored the reaction of the raw materials. After the reaction was completed, the reaction solution was cooled to below 20°C, and then used 6M The pH of the reaction system was adjusted to 3-4 with hydrochloric acid, and a solid was precipitated. After filtering, the obtained filter cake was washed once with a small amount of water, and after drying, a light yellow-green powder product (383 g) was obtained with an HPLC purity of 99.34% and a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com