Preparation method for medicine-istradefylline crystal form II for treating Parkinson
A technology for itraphylline and medicine is applied in the field of preparation of itraphylline crystal form II for treating Parkinson's disease, and can solve the problems of different stability, fluidity and compressibility, influence on drug efficacy, difference in bioavailability and the like. , to achieve the effect of improving quality, improving bioavailability, high yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add istradefylline (10g) with a purity greater than 99.6% into a mixed solvent of isopropanol (20ml) and methyl tert-butyl ether (20ml), then heat to 70°C to dissolve, and quickly cool down to 1°C after dissolution , crystallization, that is, the drug istradefylline crystal form II for the treatment of Parkinson's is obtained.
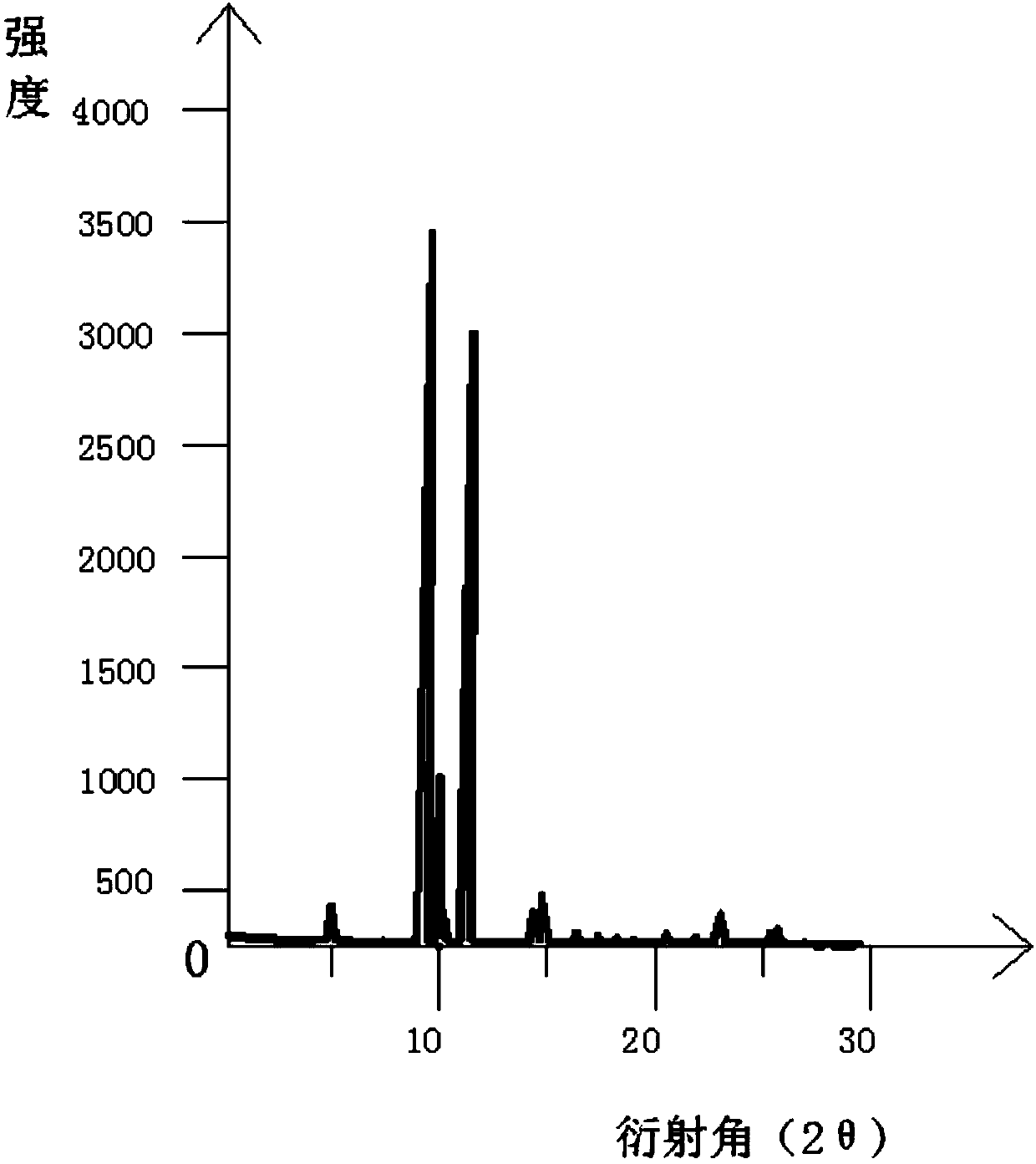
[0031] The Parkinson's drug istradefylline crystal form II prepared in Example 1 was subjected to X-ray powder diffraction test to obtain the Parkinson's drug istradefylline crystal form II at diffraction angles 2θ at 8.687 degrees, 11.683 degrees and 12.120 degrees There are obvious diffraction peaks.
[0032] The optical purity of the istradefylline crystal form II of the Parkinson's drug prepared in Example 1 was determined:
[0033] Take the istradefylline crystal form II of the drug for treating Parkinson’s prepared in Example 1, accurately weigh 100 mg and place it in a 100 ml measuring bottle, add mobile phase to ultrasonically dissolve ...
Embodiment 2
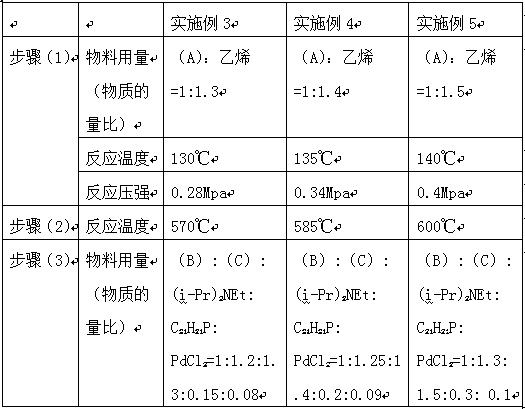
[0055] (1) Dissolve the reaction raw material 1,2-dimethoxybenzene (A) (20g, 8.2mol) and ethylene (22.5g, 9.84mol) in acetonitrile (80ml), add aluminum trichloride (2.5g) , stir well, control the reaction temperature at 127°C, and the reaction pressure at 0.2Mpa, use the liquid phase method to react 1,2-dimethoxybenzene (A) with ethylene in the tower reactor, and the reaction ends;
[0056] (2) Put the reaction solution in step (1) in a vertical reactor, add catalyst iron oxide (1.2g) and cocatalyst potassium (0.6g), add a large amount of high-temperature water vapor at 560°C, continue the reaction, and form E-2-(3,4-dimethoxy)styrene (B) (35.16g, 12.42mol);
[0057] (3) Combine E-2-(3,4-dimethoxy)styrene (B) (35.16g, 12.42mol) produced in step (2) with 1,3-diethyl-8-iodo-7 -Methylpurine-2,6-dione (C) (36.2g, 14.28mol) was mixed and dissolved in N,N-dimethylformamide (DMF) (100ml), and N,N-diisopropyl Ethylamine (40g, 14.9mol), tri-o-tolylphosphine (3.6g, 1.2mol) and palladi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com