Preparation method of istradefylline intermediate
A technology of istradefylline and intermediates, which is applied in the field of preparation of istradefylline, a drug for treating Parkinson's disease, can solve problems such as unfavorable scale-up of production, and achieve the effects of economical raw materials, convenient process operation, and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
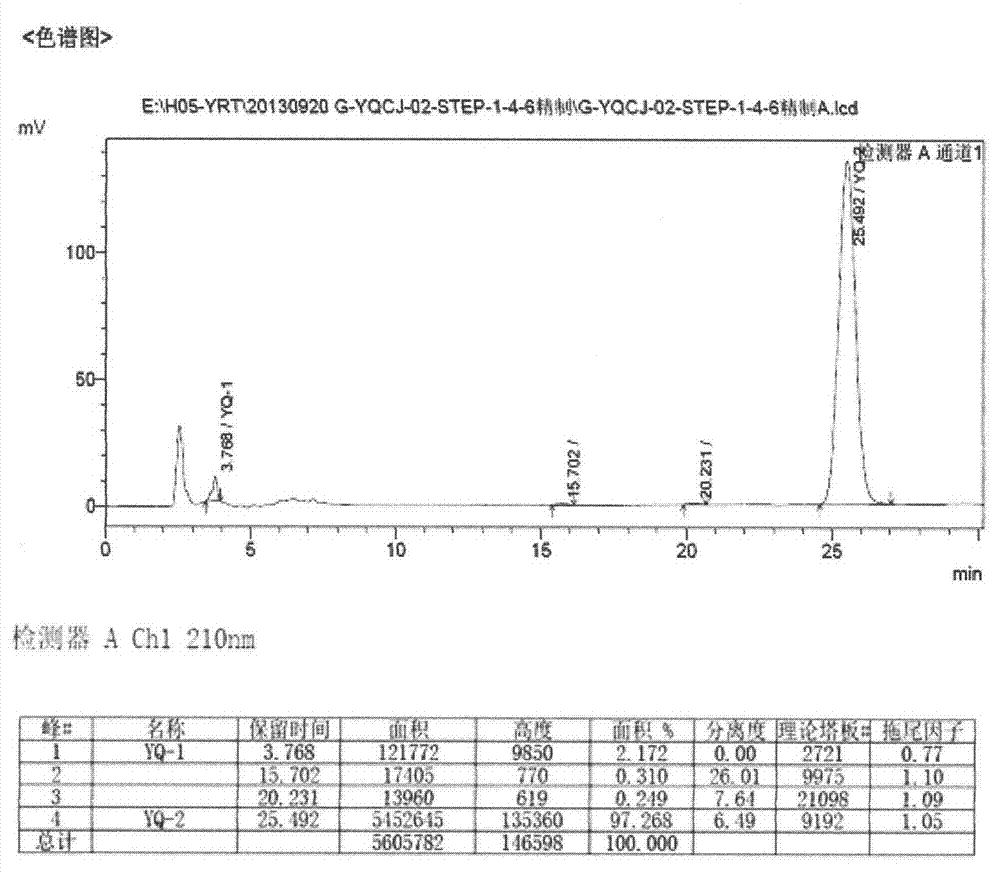
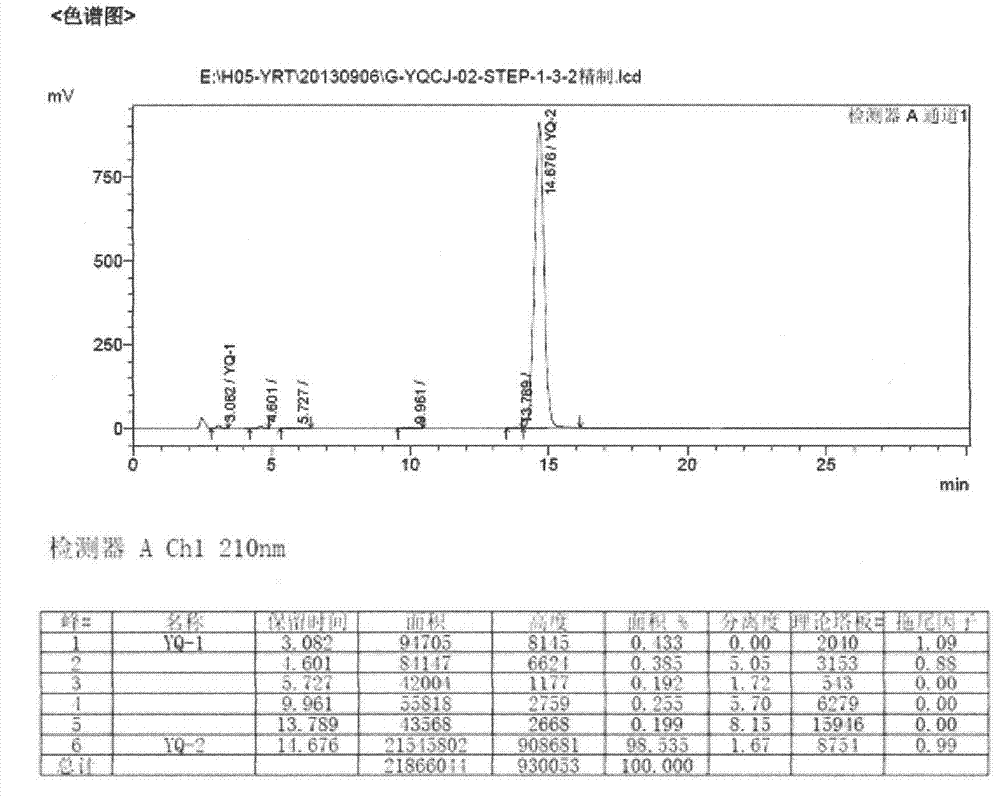
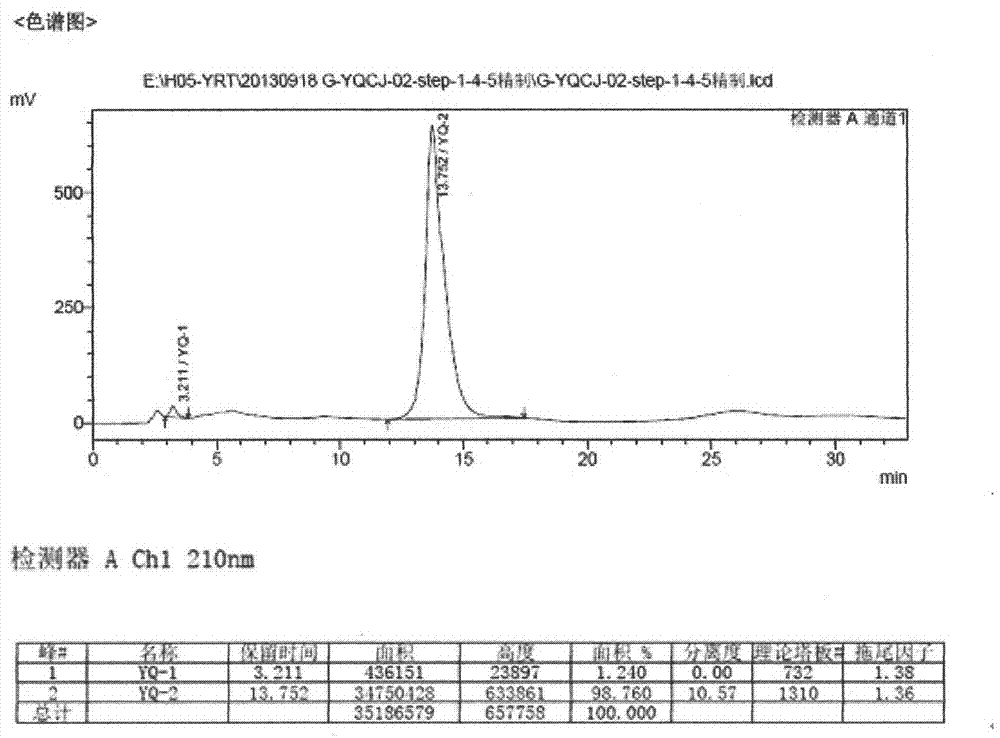
[0026] Take a clean 5000ml three-neck flask, pour 485ml of 1,4-dioxane, then pour 960ml of 1mol / L NaOH aqueous solution, and then add accurately weighed (E)-1,3-diethyl-6 amino - 100g (0.258mol) of 5-(3,4-dimethoxyphenylacryloyl)aminouracil, heat in an oil bath, raise the temperature to 110°C until reflux occurs, stir mechanically for 1 hour, and avoid the three-necked flask during the reaction. Light, HPLC to monitor the progress of the reaction. After the reaction, the temperature was lowered to about 60°C, and the reaction solution was transferred to a 5L single-necked bottle for rotary evaporation under reduced pressure at 60°C, and the rotary evaporation was stopped when a large amount of yellow solid appeared in the bottle. Transfer the paste after rotary steaming to a 5L single-necked bottle cooled with ice packs and ice water, and slowly add concentrated hydrochloric acid dropwise to adjust the pH value to 2. Suction filter the acid-adjusted paste, add about 100ml of ...
Embodiment 2
[0029] Take a clean 5000ml three-neck flask, pour 970ml of 1,4-dioxane, then pour 1920ml of 1mol / L NaOH aqueous solution, and then add accurately weighed (E)-1,3-diethyl-6 amino - 100g (0.258mol) of 5-(3,4-dimethoxyphenylacryloyl)aminouracil, heat in an oil bath, raise the temperature to 115°C until reflux occurs, stir mechanically for 2 hours, and avoid the three-necked flask during the reaction. Light, HPLC to monitor the progress of the reaction. After the reaction, the temperature was lowered to about 60°C, and the reaction solution was transferred to a 5L single-necked bottle for rotary evaporation under reduced pressure at 70°C, and the rotary evaporation was stopped when a large amount of yellow solid appeared in the bottle. Transfer the paste after rotary steaming to a 5L single-necked bottle cooled with ice packs and ice water, and slowly add concentrated hydrochloric acid dropwise to adjust the pH value to 2. Suction filter the acid-adjusted paste, add about 100ml o...
Embodiment 3
[0032] Take a clean 10000ml three-neck flask, pour 1450ml of 1,4-dioxane, then pour 2880ml of 1mol / L NaOH aqueous solution, and then add accurately weighed (E)-1,3-diethyl-6 amino - 100g (0.258mol) of 5-(3,4-dimethoxyphenylacryloyl)aminouracil, heat in an oil bath, raise the temperature to 125°C until reflux occurs, stir mechanically for 4 hours, and avoid the three-necked flask during the reaction. Light, HPLC to monitor the progress of the reaction. After the reaction, the temperature was lowered to about 60°C, and the reaction solution was transferred to a 5L single-necked bottle for rotary evaporation under reduced pressure at 80°C, and the rotary evaporation was stopped when a large amount of yellow solid appeared in the bottle. Transfer the paste after rotary steaming to a 5L single-necked bottle cooled with ice packs and ice water, and slowly add concentrated hydrochloric acid dropwise to adjust the pH value to 2. The paste that has adjusted acid is suction filtered, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com