Preparation method of istradefylline intermediate
A technology of istradefylline and intermediates, which is applied in the field of drug synthesis, can solve the problems of product loss and low purity, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
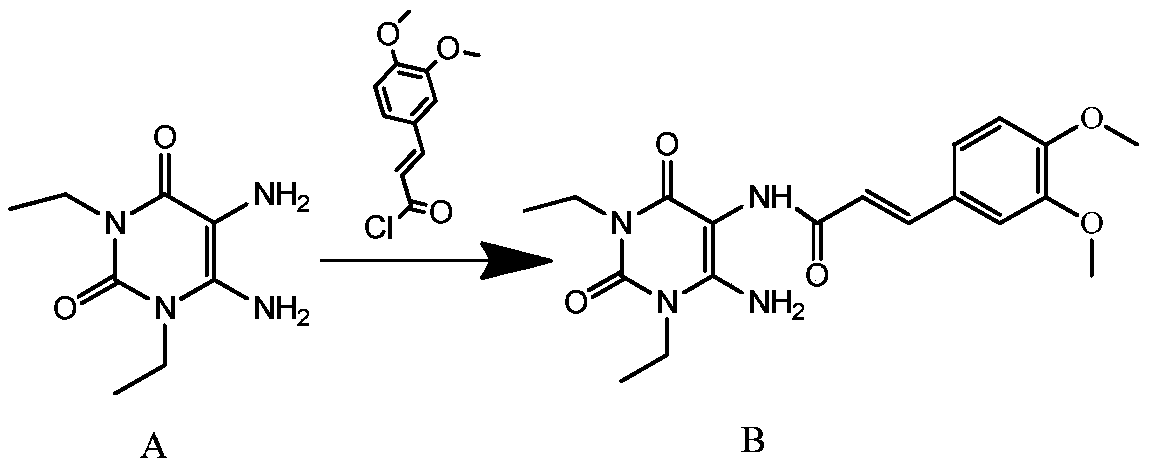
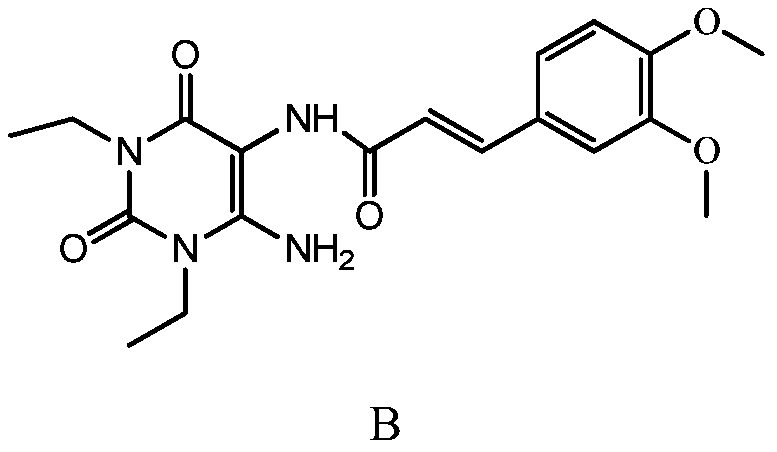
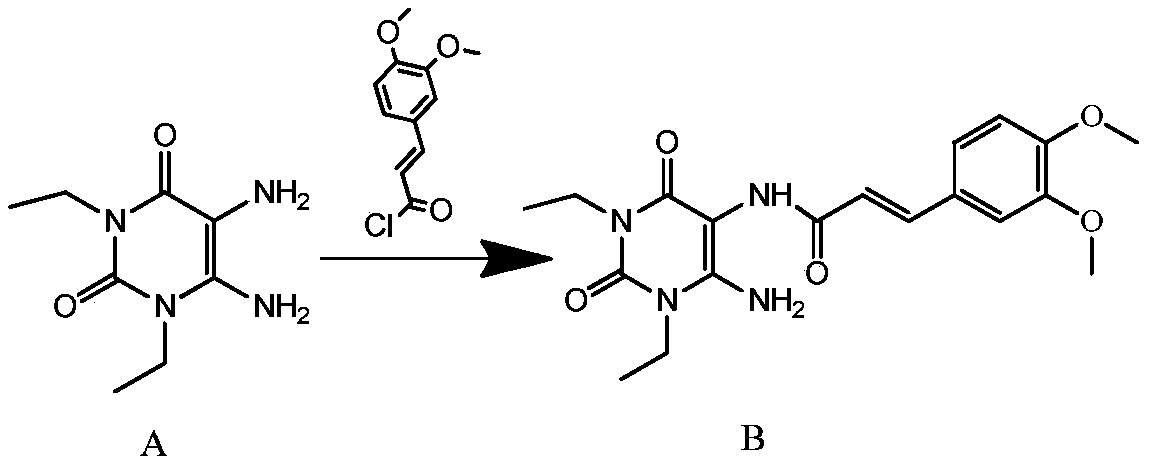
[0028] In a clean and dry reaction flask, put 30g (0.15mol) of 1,3-diethyl-5,6-diaminouracil, 1200ml of dichloromethane, and 60g (0.59mol) of triethylamine into a complete solution. 15~20℃, slowly add 50g (0.22mol) (E)-3,4-dimethoxyphenylacryloyl chloride, TLC detects the reaction end point, after the reaction is complete, add 1500ml of 5% sodium carbonate solution to the reaction solution , stirred for 10 minutes to completely neutralize the generated hydrochloric acid, let stand to separate layers, and removed the lower organic layer; added 1200ml of purified water to the organic layer, cooled the reaction solution to 3-7°C, and stirred for 1 hour. The reaction solution was filtered, and the filter cake was (E)-1,3-diethyl-6-amino-5-(3,4-dimethoxyphenylacryloyl)aminouracil, 53.0g, off-white solid, The purity is greater than 98%, and the yield is 90.2%.
Embodiment 2
[0030] In a clean and dry reaction flask, put 30g (0.15mol) of 1,3-diethyl-5,6-diaminouracil, 900ml of dichloromethane, and 60g (0.60mol) of triethylamine into a complete solution. Cool down to 10°C to 15°C, slowly add 50g (0.22mol) (E)-3,4-dimethoxyphenylacryloyl chloride, TLC detects the end of the reaction, after the reaction is complete, add 600ml of 5% Sodium carbonate solution, stirred for 10 minutes to completely neutralize the generated hydrochloric acid, let stand to separate layers, and removed the lower organic layer; added 900ml of purified water to the organic layer, cooled the reaction solution to 0-2°C, and stirred for 1 hour. The reaction liquid was filtered, and the filter cake was (E)-1,3-diethyl-6-amino-5-(3,4-dimethoxyphenylacryloyl)aminouracil, 51.8g, light yellow solid, The purity is greater than 98%, and the yield is 88.1%.
Embodiment 3
[0032] In a clean and dry reaction flask, put 30g (0.15mol) of 1,3-diethyl-5,6-diaminouracil, 1500ml of dichloromethane, and 60g (0.60mol) of triethylamine into a complete solution. Adjust the temperature to 20-25°C, slowly add 50g (0.22mol) (E)-3,4-dimethoxyphenylacryloyl chloride, TLC to detect the end of the reaction, after the reaction is complete, add 2400ml of 5% Sodium carbonate solution, stirred for 10 minutes to completely neutralize the generated hydrochloric acid, let stand to separate layers, and removed the lower organic layer; added 1500ml of purified water to the organic layer, cooled the reaction solution to 10-12°C, and stirred for 1 hour. The reaction solution was filtered, and the filter cake was (E)-1,3-diethyl-6-amino-5-(3,4-dimethoxyphenylacryloyl)aminouracil, 50.9g, off-white solid, The purity is greater than 98%, and the yield is 86.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com