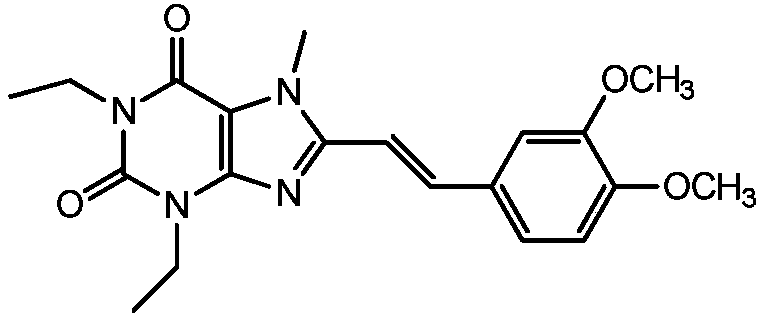
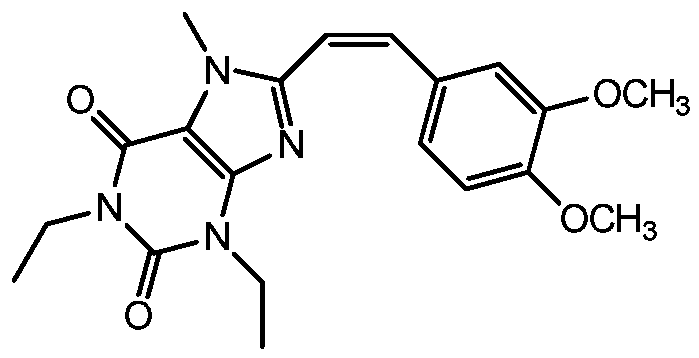
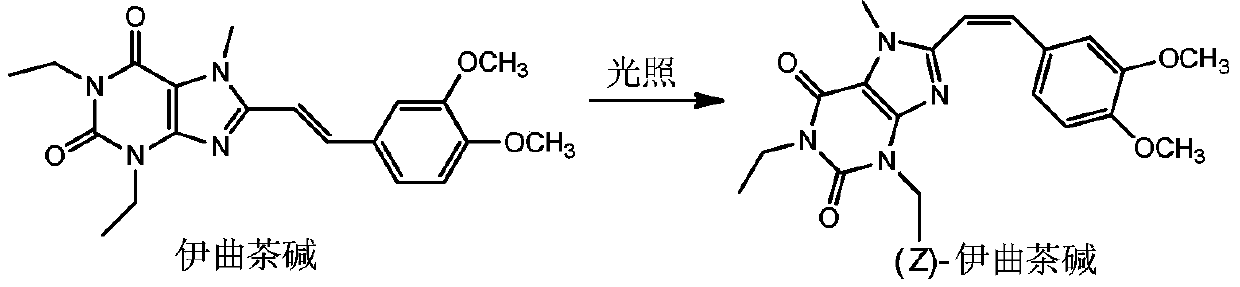
Method for preparing (Z)-istradefylline
A technology of istradefylline and acetonitrile, applied in the field of medicinal chemistry, can solve the problems of cumbersome operation, waste of materials, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Weigh 100 mg of istradefylline into a test tube, and add 20 ml of solvent (acetonitrile-water=4:1 (volume ratio)). Close the lid tightly, shake to dissolve, and place under sunlight for 1 to 1.5 hours;
[0030] (2) Separation and preparation are carried out by reverse high performance liquid chromatography preparation method, and the elution fraction of (Z)-istradefylline is collected;
[0031] The conditions of the reverse high performance liquid chromatography preparative method are:
[0032] Sample: solution after light treatment
[0033] Chromatographic column: Hanbang Dubhe C18, Φ30×250mm, 10um
[0034] Detection wavelength: 355nm
[0035] Mobile phase: acetonitrile-water=70:30 (volume ratio)
[0036] Flow rate: 30ml / min
[0037] Injection volume: 5ml;
[0038] (3) The collected solution was evaporated to remove acetonitrile under reduced pressure at 30-35°C, transferred to a petri dish, put into a lyophilizer and freeze-dried according to the procedure t...
Embodiment 2
[0040] (1) Weigh 100 mg of istradefylline into a test tube, and add 20 ml of solvent (acetonitrile-water=3:1 (volume ratio)). Close the lid tightly, shake to dissolve, and place under sunlight for 1 to 1.5 hours;
[0041] (2) Separation and preparation are carried out by reverse-phase high-performance liquid chromatography preparation method, and the elution fraction of (Z)-istradefylline is collected; the conditions of the reverse-phase high-performance liquid chromatography preparation method are the same as in Example 1;
[0042] (3) The collected liquid was evaporated to remove acetonitrile under reduced pressure at 25-30°C, transferred to a petri dish, put into a lyophilizer and freeze-dried according to the procedure to obtain 63 mg of the target substance, HPLC: 99.5% (area normalization method) .
Embodiment 3
[0044] (1) Weigh 100 mg of istradefylline into a test tube, and add 20 ml of solvent (acetonitrile-water=2:1 (volume ratio)). Close the lid tightly, shake to dissolve, and place under sunlight for 1 to 1.5 hours;
[0045] (2) Separation and preparation are carried out by reverse-phase high-performance liquid chromatography preparation method, and the elution fraction of (Z)-istradefylline is collected; the conditions of the reverse-phase high-performance liquid chromatography preparation method are the same as in Example 1;
[0046] (3) The collected liquid was evaporated to remove acetonitrile under reduced pressure at 28-32°C, transferred to a petri dish, put into a lyophilizer and freeze-dried according to the procedure to obtain 59 mg of the target substance, HPLC: 99.0% (area normalization method) .
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com