Istradefylline raw material drug and preparation method thereof
A technology of istraphylline and bulk drug, applied in the field of istraphylline bulk drug and preparation thereof, can solve the problems of not effectively reducing or avoiding the side effects of istraphylline and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0056] The following examples are used to further describe the present invention, but these examples do not limit the scope of the present invention.
[0057] The experimental methods not indicating specific conditions in the examples of the present invention are generally in accordance with conventional conditions, or in accordance with the conditions suggested by raw material or commodity manufacturers. Reagents without specific sources indicated are conventional reagents purchased in the market.
Embodiment 1
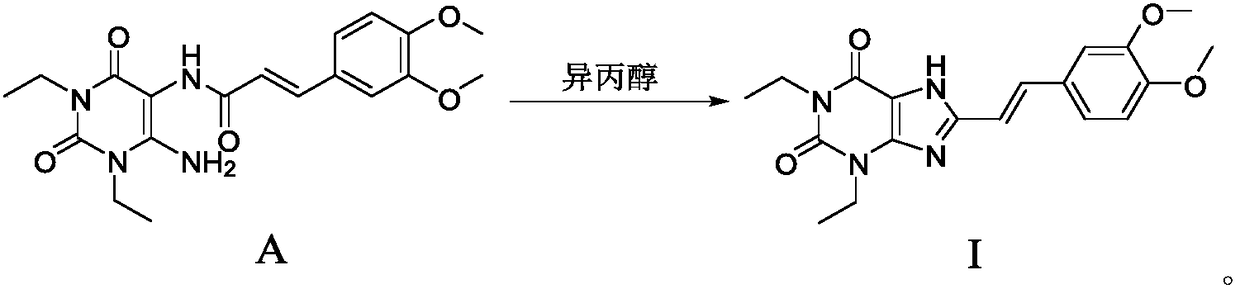
[0058] Example 1, E-N-(6-amino-1,3-diethyl-2,4-dione-1,2,3,4-tetrahydropyrimidin-5-yl)-3-(3,4- Preparation of dimethoxy) propionamide (compound A)
[0059]
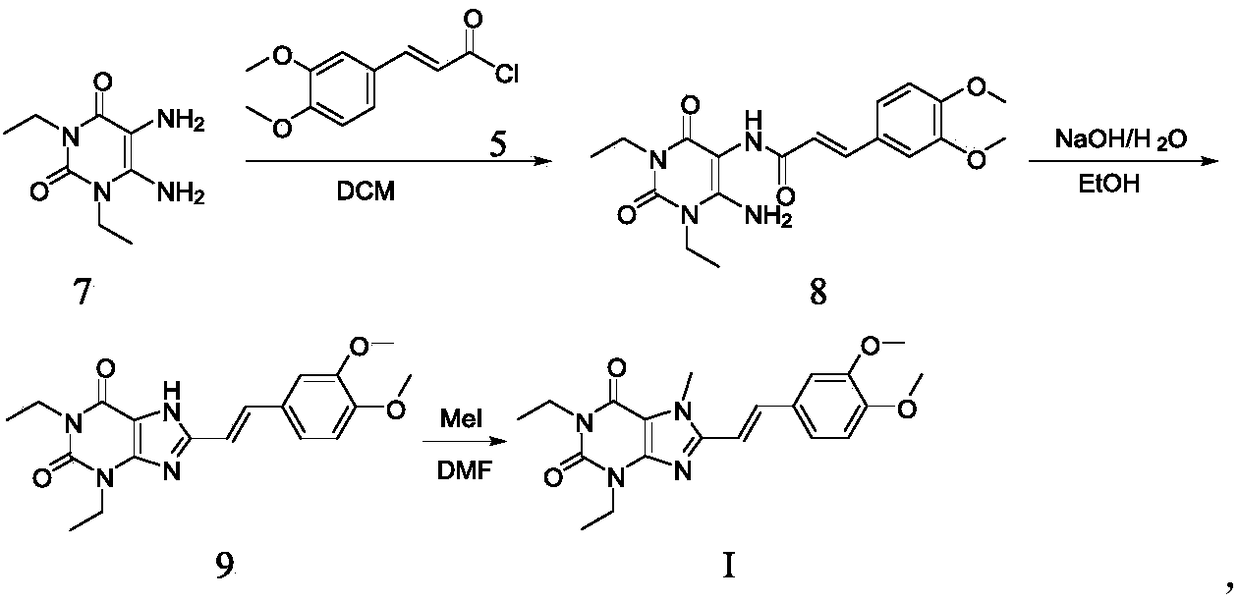
[0060] Suspend the compound 1,3-diethyl-5,6-diaminouracil (1.0kg, prepared according to the method of EP0590919A1) of formula 1 in dichloromethane, add pyridine (1.6kg) under stirring, dissolve the dichloro Compound 2 (1.54kg) in methane (3.5L) was added dropwise to the reaction system, and the dropwise addition was completed in about 1.5 hours. After stirring at room temperature for 15-20 hours, a saturated aqueous sodium bicarbonate solution was added dropwise to the reaction solution to adjust the pH to Around 7-8, solids are precipitated. After filtration, the filter cake was washed twice with water, and the filter cake was dried to obtain a light yellow powdery solid product (1.56 kg), with a yield of 79.5%.
Embodiment 2
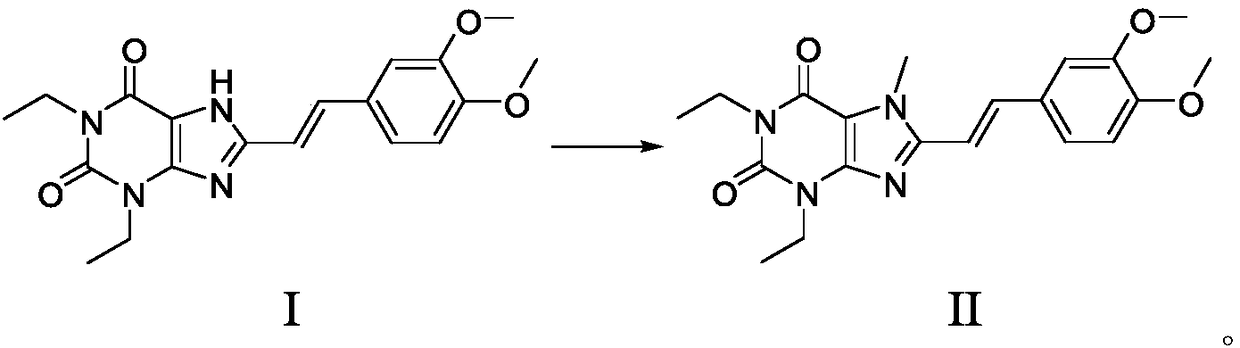
[0061] Example 2. Preparation of 8-((E)-2-(3,4-dimethoxyphenyl)vinyl)-1,3-diethyl-3,7-dihydro-1H-purine-2 , the preparation of 6-diketone (compound of formula I)
[0062]
[0063] Method A:
[0064] Formula A compound (500g, 1.29mol) was added in 10L reactor, then added isopropanol (2.5L), stirred, sodium hydroxide solid (486g, 12.15mol) was dissolved in water (2.0L), then stirred Add it dropwise to the reaction system quickly, and the dropwise addition is completed in about 30 minutes. The temperature of the reaction is raised to 75-80°C. After 8-9 hours of reaction, TLC monitors that the raw materials have reacted completely, and the reaction solution is cooled to below 20°C, and then heated with 6M Hydrochloric acid was used to adjust the pH of the reaction system to 3-4, and a solid was precipitated. After filtering, the resulting filter cake was washed once with a small amount of water, and dried to obtain a light yellow-green powder product (383 g), with an HPLC pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com