Preparation method for removing methyl impurities from istradefylline
A technology for istradefylline and demethylation, which is applied in the field of synthesis of istradefylline demethylation impurities, can solve problems such as shortages, and achieve the effects of high impurity purity, high efficiency, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
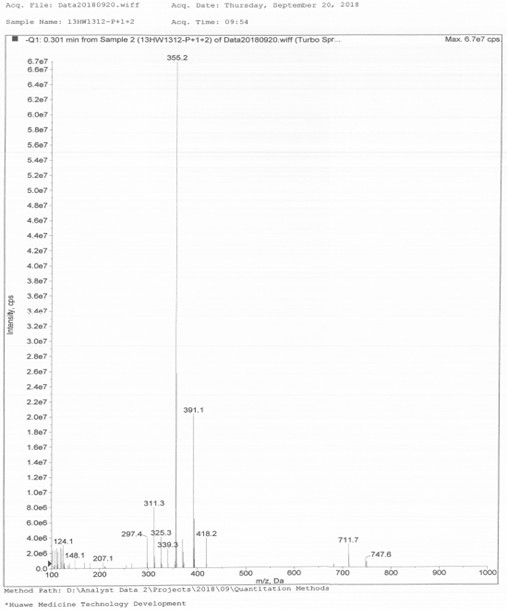
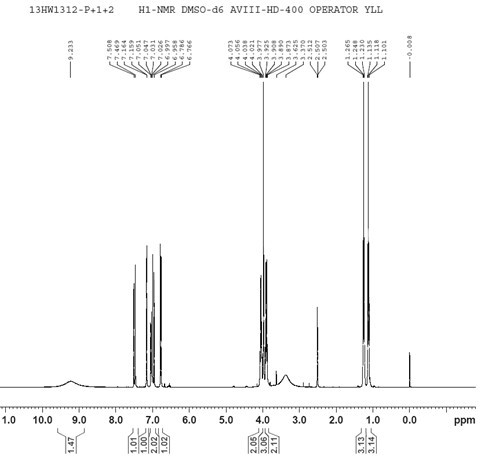
[0044] Preparation of 8-[(E)-2-(3,4-dihydroxyphenyl)ethenyl]-1,3-diethyl-7-methylpurine-2,6-dione, the compound of formula I method, including the following steps:
[0045] Heat 10g istradefylline, 30g hydrobromic acid aqueous solution (mass fraction 48%), and 2mL water to 120°C (±5°C), the system is dissolved, and the reaction is monitored by TLC (developer: V (petroleum ether) / V (Ethyl acetate) = 1 / 8, Rf (Itraphylline) = 0.8, Rf (I) = 0.6), react for 3~4h and cool down to 0°C~10°C, stir and crystallize, filter, and use for filter cake After rinsing with an appropriate amount of water, blow air at 50°C (±2°C) and dry to constant weight to obtain 7.6g of the crude product of formula I;
[0046] Take 7.6g of the crude product of the above formula I, 22mL of N'N-dimethylformamide, stir and heat up to 85°C~90°C, add 12mL of water dropwise, after dropping, cool down to 20°C~30°C, stir and crystallize for 12h, filter, The filter cake was rinsed with an appropriate amount of water...
Embodiment 2
[0057]8-[(E)-2-(3-Hydroxy-4-dimethoxyphenyl)vinyl]-1,3-diethyl-7-methylpurine-2,6-dione (Formula II ) and 8-[(E)-2-(3-methoxy-4-hydroxyphenyl)vinyl]-1,3-diethyl-7-methylpurine-2,6-dione (formula III) Preparation method:
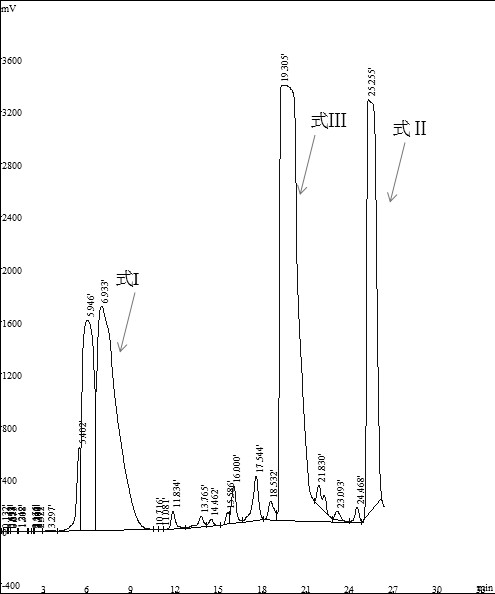
[0058] Take 4g (11.2mmol) of the compound of formula I, 25mL of dichloromethane (DCM), 2.2g of potassium carbonate (16.1mmol), add 2.38g of iodomethane (16.8mmol), and react at 25~30°C for 12~13h. Monitor the reaction by TLC (developing solvent: V (dichloromethane) / V (methanol) = 15 / 1, Rf (I) = 0.4~0.5, Rf (II) = 0.7, Rf (III) = 0.6), the reaction is over After filtering, the filtrate was concentrated under reduced pressure and the solvent was evaporated (concentration conditions: water bath temperature 30°C, pressure -0.1MPa) to obtain 3.9g concentrate;
[0059] Take 3 g of the above-mentioned concentrate, add 18 mL of methanol for ultrasonic dissolution, filter out a little insoluble matter, and separate according to the liquid phase separation method in ...
Embodiment 3
[0061] The preparation method of formula II and formula III compound, comprises the following steps:
[0062] Add 12g (33.6mmol) of the compound of formula I, 78mL N,N-dimethylformamide (DMF), 6.7g potassium carbonate (48.4mmol), and add 7.15g iodomethane (50.4mmol) in three batches. React at 30°C for 4.5~5h, during which the reaction can be monitored by TLC (developer: V (dichloromethane) / V (methanol)=15 / 1, Rf(I)=0.4~0.5, Rf(II)=0.7, Rf(III)=0.6), after the reaction, concentrated under reduced pressure to remove the solvent (concentration conditions: water bath temperature 55°C, pressure -0.1MPa), and obtained 25.1g of yellow oil;
[0063] Add 250mL tetrahydrofuran to the above oil, stir and heat up to 50~55°C, continue to stir for 0.5h, then concentrate the filtrate under reduced pressure (concentration conditions: water bath 45°C, pressure -0.1MPa) to obtain 14.1g of concentrate;
[0064] Take 5g of the above-mentioned concentrate, add 30mL of methanol for ultrasonic disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com