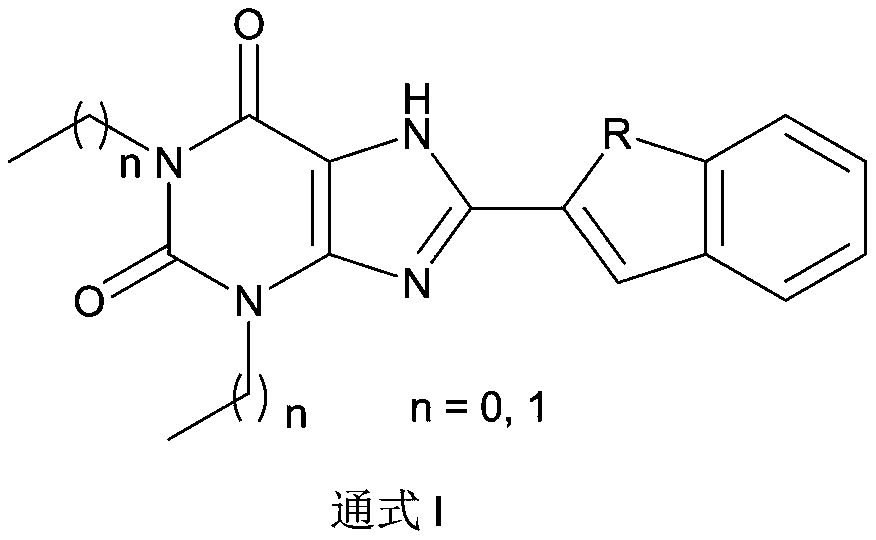
Xanthine derivative as well as preparation method and application thereof
A derivative, xanthine technology, applied in the field of medicine, can solve the problems of fast conversion, poor photosensitivity, and no reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of 8-(benzo[b]thiophen-2-yl)-1,3-dimethyl-1H-purine-2,6(3H,7H)-dione (Compound 1):
[0048] 1mmol of intermediate A (5,6-diamino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione) and 1mmol of EDC (1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride) was sequentially added to the reaction flask, 5mL of dioxane and 5mL of water were added, and 1mmol of benzo[b]thiophene-2-carboxylic acid was added under stirring at room temperature, to the reaction solution 1M HCl aqueous solution was added dropwise to adjust the pH=5~6, and the amide compound was reacted under stirring at room temperature; the reaction was detected by TLC (developing agent was chloroform:methanol=20:1). Heat up to reflux reaction; TLC detection reaction (developing agent is chloroform:methanol=20:1), after the reaction is completed, use 1M hydrochloric acid aqueous solution to adjust PH=5~6, precipitate a large number of solids, filter and wash with water, ethanol recrystallization to obtain c...
Embodiment 2
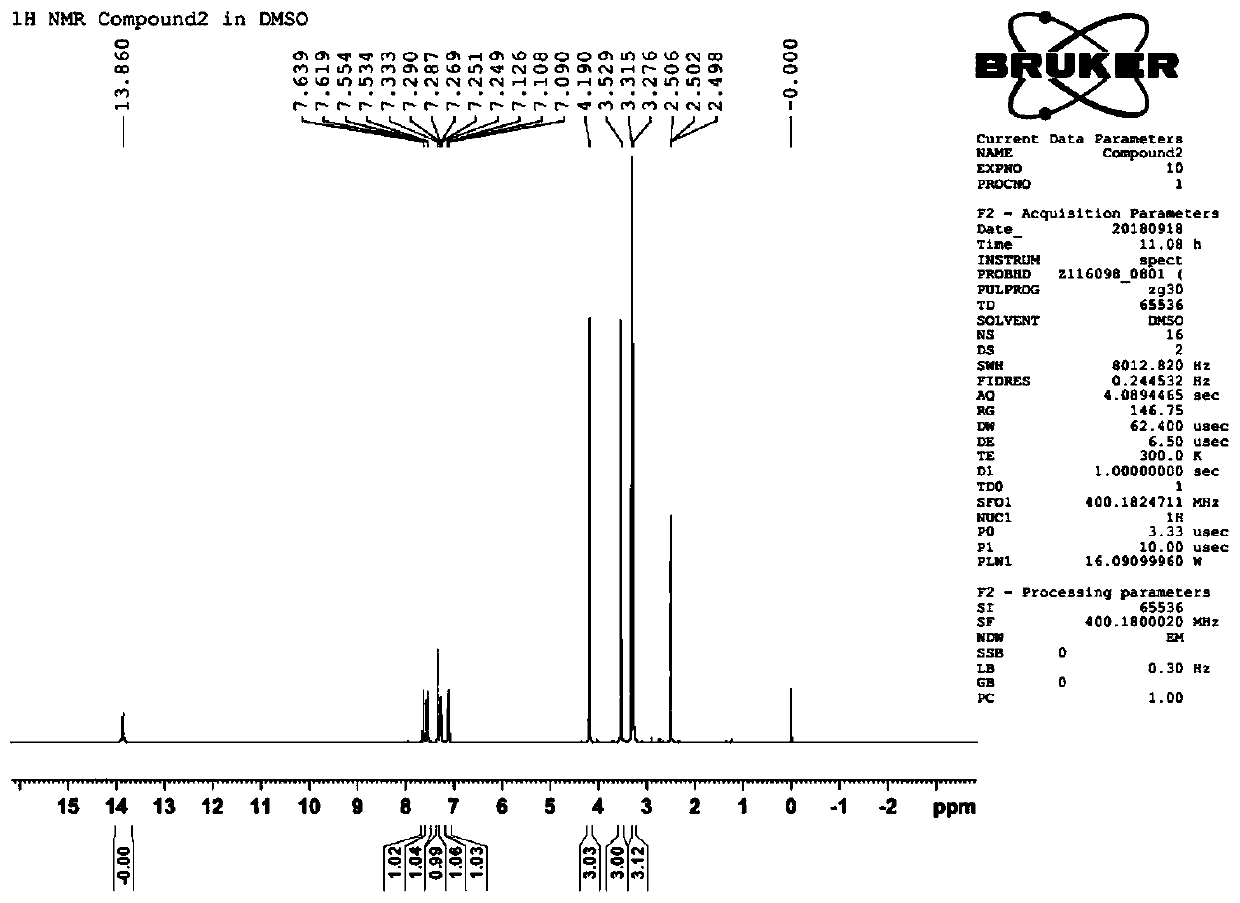
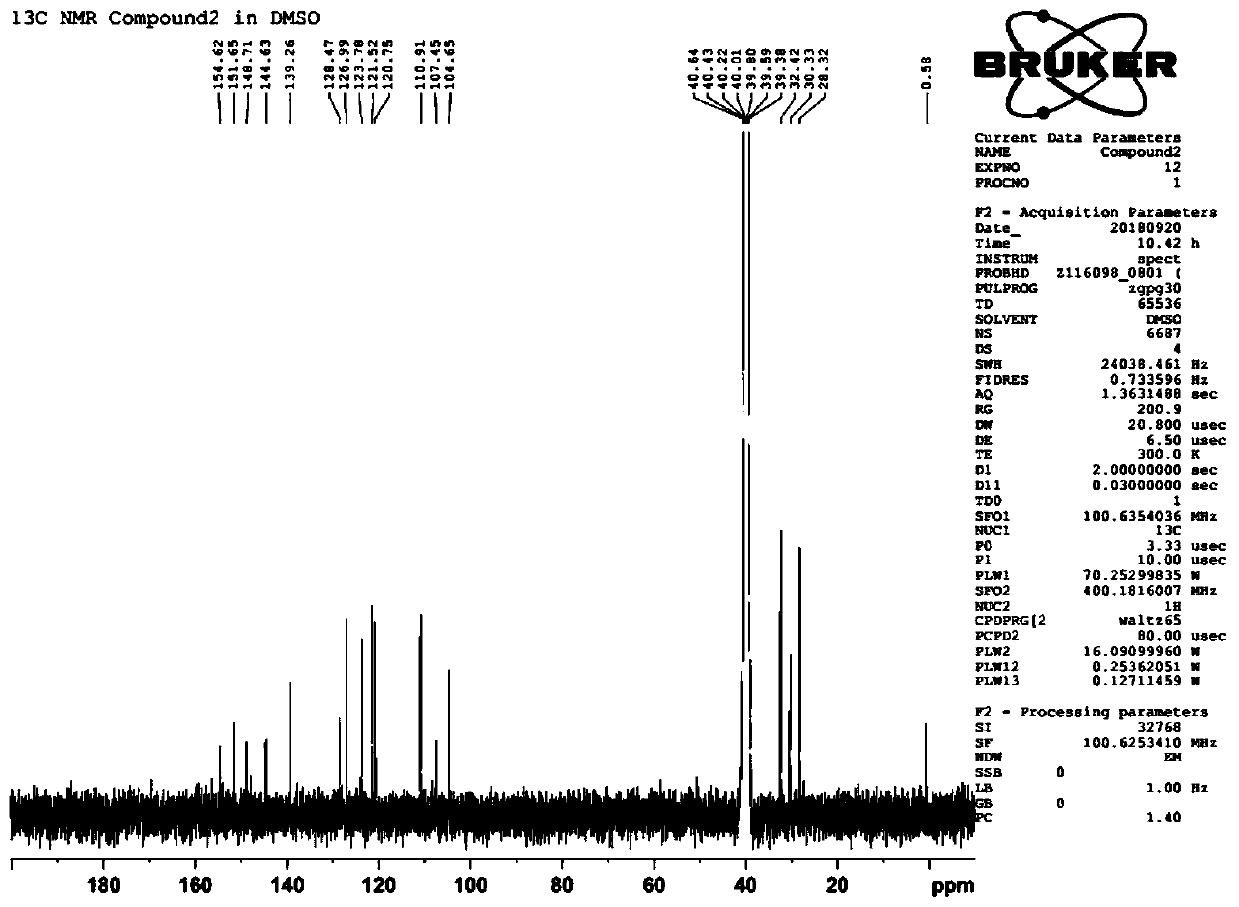
[0050] Preparation of 1,3-dimethyl-8-(1-methyl-1H-indol-2-yl)-1H-purine-2,6(3H,7H)-dione (compound 2):
[0051]1mmol of intermediate A (5,6-diamino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione) and 1.3mmol of EDC (1-ethyl-(3-di Methylaminopropyl) carbodiimide hydrochloride) was added to the reaction flask in turn, 5 mL of dioxane and 5 mL of water were added, and 1.2 mmol of 1-methyl-1H-indole-2-carboxylic acid was added under stirring at room temperature , in the reaction solution, dropwise add 1M HCl aqueous solution to adjust PH=5~6, react under stirring at room temperature to generate amide compound; and 1mL water, warming up to reflux reaction; TLC detection reaction (developing agent is chloroform:methanol=20:1), after the completion of the reaction, use 1M hydrochloric acid aqueous solution to adjust the pH=5~6, a large amount of solids are precipitated, filtered and washed with water, and recrystallized from ethanol to obtain Compound 2; 1H-NMR (400MHz, DMSO-d6): δ3.28(s, ...
Embodiment 3
[0053] Preparation of 1,3-diethyl-8-(1-methyl-1H-indol-2-yl)-1H-purine-2,6(3H,7H)-dione (compound 3):
[0054] 1mmol of intermediate A (5,6-diamino-1,3-diethylpyrimidine-2,4(1H,3H)-dione) and 1mmol of EDC (1-ethyl-(3-dimethyl Aminopropyl) carbodiimide hydrochloride) was sequentially added to the reaction flask, 5 mL of dioxane and 5 mL of water were added, and 1 mmol of 1-methyl-1H-indole-2-carboxylic acid was added under stirring at room temperature. In the reaction solution, 1M HCl aqueous solution was added dropwise to adjust the pH=5~6, and the amide compound was reacted under stirring at room temperature; the reaction was detected by TLC (developing agent was chloroform:methanol=20:1), after the reaction was completed, 1.5mmol of NaOH solid and Heat 1mL of water to 110°C for reflux reaction; TLC detection reaction (developing solvent: chloroform:methanol=20:1), after the completion of the reaction, use 1M hydrochloric acid aqueous solution to adjust the pH to 5-6, a large...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com