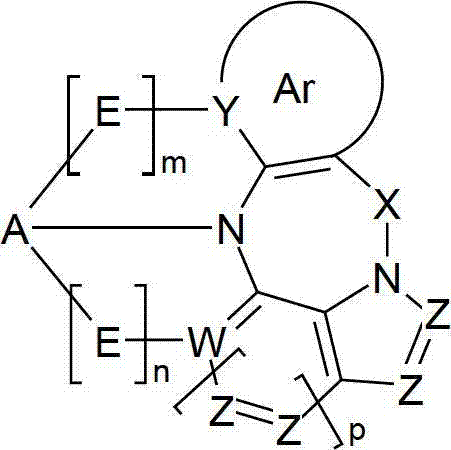
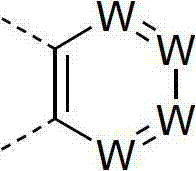
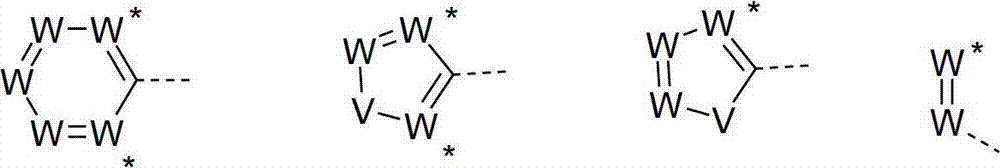
Materials for organic electroluminescence devices
A conditional and group technology, applied in the direction of organic dyes, electrical solid devices, electrical components, etc., can solve the problems of strong oxidation sensitivity, damage to material preparation, purification and storage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0167]
[0168] 37.4 g (145 mmol) of 11,12-dihydroindolo[2,3-a]carbazole were added to 23 ml (159.5 mmol) of methyl 2-iodobenzoate in 150 ml of di-n-butyl ether , and degas the solution. Then 10g (158mmol) of copper powder, 1.38g (7mmol) of copper iodide (I) and 22g (159.6mmol) of K 2 CO 3 This was added to the mixture, and the mixture was stirred at 144° C. for 4 days under protective gas. Use MgSO 4 The organic phase was dried and the solvent was removed under vacuum. The residue was recrystallized from acetone and finally sublimed in high vacuum. Yield: 20.9 g (58.4 mmol), 40% of theory, 99.9% pure according to HPLC.
Embodiment 2
[0170]
[0171] By the same procedure as in Example 1, by making 37.4g (145mmol) 11,12-dihydroindolo[2,3-a]carbazole and 54g (159.5mmol) 3-iodobiphenyl-2- Carboxylic acid methyl ester reaction synthesis of the compound. Convert the residue from CH 2 Cl 2 / isopropanol for recrystallization, and finally sublimation in high vacuum. Yield: 24.5 g (56 mmol), 39% of theory, 99.9% pure according to HPLC.
Embodiment 3
[0173]
[0174] By the same procedure as in Example 1, by making 37.4g (145mmol) of 11,12-dihydroindolo[2,3-a]carbazole and 42g (159.5mmol) of 3-iodothiophene-2-carboxyl Acid methyl ester reaction synthesizes said compound. Convert the residue from CH 2 Cl 2 / isopropanol for recrystallization, and finally sublimation in high vacuum. Yield: 22.6 g (62 mmol), 43% of theory, 99.9% pure according to HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com