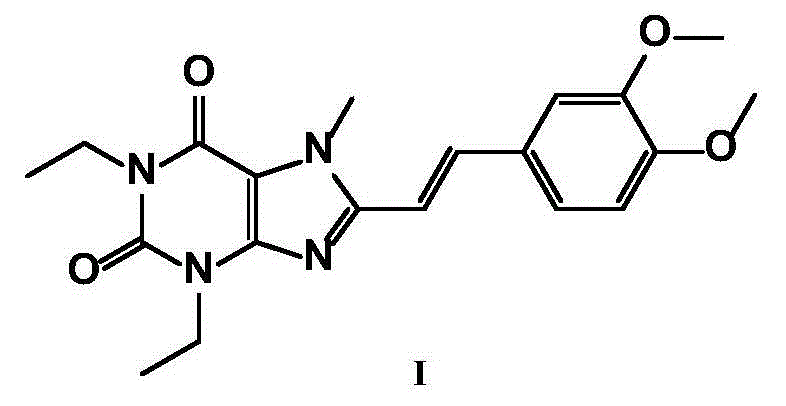
New crystal form of istradefylline and preparation method thereof
A technology of istradefylline and crystal form, which is applied in the field of new crystal form of istradefylline and its preparation, can solve problems such as crystal aggregation and uneven dispersion, and achieve the effects of improving bioavailability, small particle size, and good properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0044] Preparation example 1: the preparation method of istradefylline crude product
[0045]
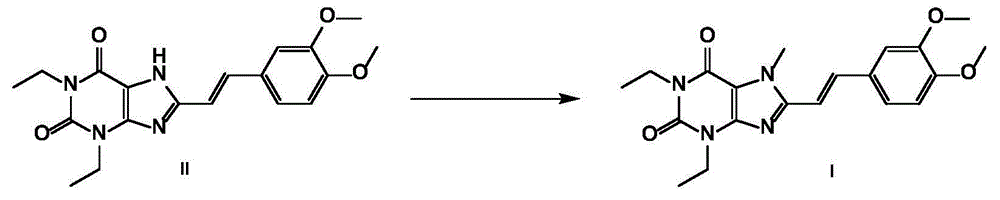
[0046] Under mechanical stirring, add intermediate II 50g (0.135mol), potassium carbonate 28g (0.203mol), N,N-dimethylformamide 550mL and methyl iodide 15mL (0.241mol) into a 3L reaction flask, heat to 50°C, The reaction was stirred for 5 h, cooled to 0°C, and 550 mL of purified water was added to the reaction solution. After filtering, the filter cake was washed with 1.1 L of purified water, and then vacuum-dried at 45° C. to obtain 51.3 g of a light yellow solid with a yield of 99% and a HPLC purity of 98.6%, which was used for the preparation of the following istradefylline A crystal form.
Embodiment 1
[0047] Embodiment 1: Preparation of istradefylline A crystal form
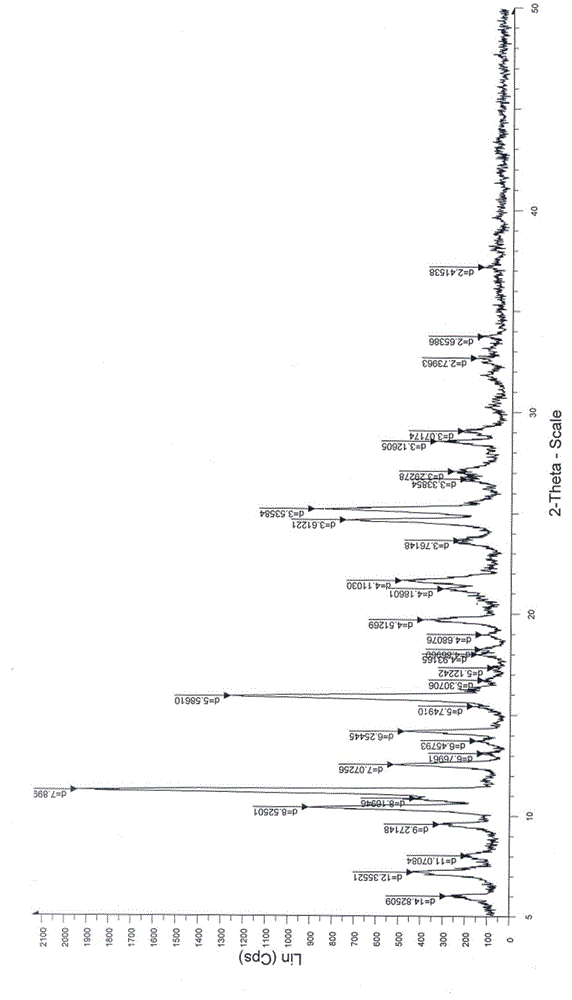
[0048] Add 3 g (7.8 mmol) of crude istradefylline to 60 mL of dichloromethane, stir to dissolve. At room temperature, add 60 mL of n-heptane, then cool to 0-10°C, stir and crystallize for 2 hours. Filtrate and dry under vacuum at 45°C to obtain 2.67g of light yellow-green solid powder, yield 89%, HPLC purity 99.8%, product particle size D 90 It is 40.231 μm. The obtained crystalline sample is subjected to powder X-ray powder diffraction, and the obtained collection of illustrative plates is shown in the attached figure 1 , and the spectral data are shown in Table 1.
[0049] Table 1 The characteristic peak data of istradefylline A crystal form powder X-ray diffraction obtained in Example 1
[0050] 2θ(°) Relative Strength(%) 6.0 14.4 7.1 22.1 9.5 16.1 10.4 46.6 11.2 100 12.5 26.7 14.1 24.3 15.8 64.7 19.7 20 21.6 25.2 24.6 38.2 25.2 45....
Embodiment 2~11
[0051] Examples 2-11: Investigation on preparation conditions of crystal form A of istradefylline
[0052] With reference to the preparation method of Example 1, only the corresponding preparation conditions are changed, and the experimental conditions and results are as follows:
[0053] Table 2 Experimental results of Examples 2 to 11
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com