Preparation method and use of istradefylline
A technology of istradefylline and its compounds, which is applied in the field of preparation of istradefylline, can solve the problems of large-scale production of istradefylline, complex process, high product cost, etc., and achieve low production cost, simple synthetic route, The effect of high production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
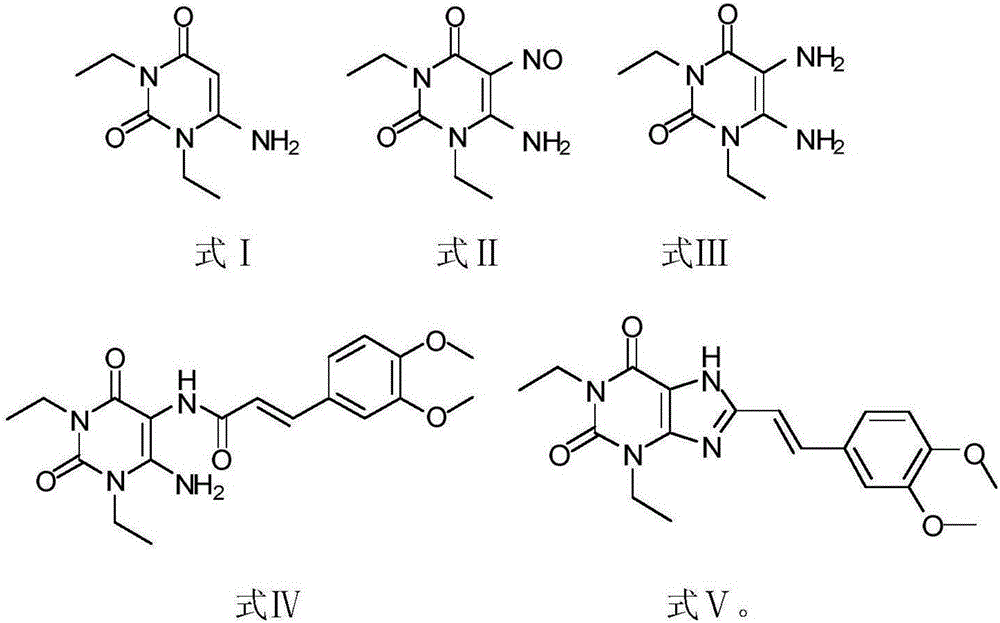
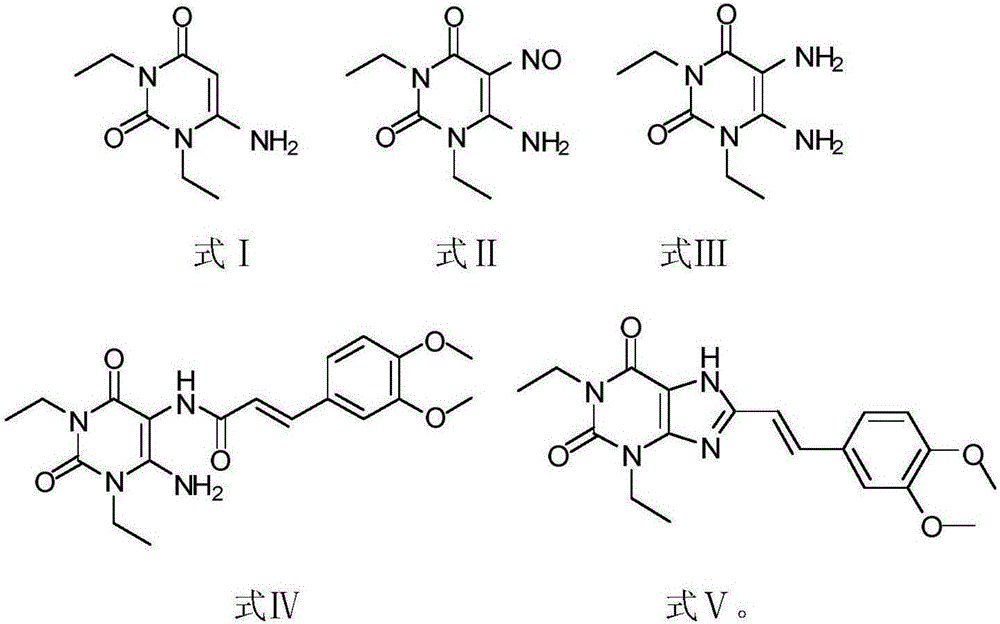
[0023] 1) Put 180g of 1,3-diethylformamide and 125g of cyanoacetic acid in 185ml of acetic anhydride for ring formation reaction. The reaction condition is to heat and reflux at 78°C for 2 hours. After the reaction is completed, add sodium hydroxide, adjust the pH to neutral and pump filtered, washed three times with water, and the resulting white solid was compound Ⅰ;
[0024] 2) Add 260 g of compound I to 1 L of acetic acid, stir for 30 minutes, and then add sodium nitrite for nitration reaction. The reaction time is 1.5 hours. After the reaction is completed, the solid obtained by suction filtration and drying is compound II;
[0025] 3) Pass hydrogen through 260 g of compound II and 2.2 L of methanol for reduction reaction. The reaction time is 20 h. After the reaction is completed, the yellow solid obtained by HPLC detection and spin-drying is compound III;
[0026] 4) Under dark and oxygen-free conditions, 150g of compound III, 200g of 3-(3,4-dimethoxy-phenolyl)-acryloyl...
Embodiment 2
[0030] 1) Put 180g of 1,3-diethylformamide and 120g of cyanoacetic acid in 180ml of acetic anhydride for ring formation reaction. The reaction condition is to heat and reflux at 75°C for 1.5h. After the reaction is completed, add sodium hydroxide to adjust the pH to neutral After suction filtration and washing with water three times, the obtained white solid was compound Ⅰ;
[0031] 2) Add 260 g of compound I to 1 L of acetic acid, stir for 30 minutes, and then add sodium nitrite for nitration reaction. The reaction time is 1. After the reaction is completed, the solid obtained by suction filtration and drying is compound II;
[0032] 3) Pass hydrogen through 260 g of compound II and 2.5 L of methanol for reduction reaction. The reaction time is 24 hours. After the reaction is completed, the yellow solid obtained by HPLC detection and spin-drying is compound III;
[0033] 4) Under dark and oxygen-free conditions, 150g of compound III, 200g of 3-(3,4-dimethoxy-phenolyl)-acryloy...
Embodiment 3
[0037] 1) Put 180g of 1,3-diethylformamide and 122g of cyanoacetic acid in 182ml of acetic anhydride for ring formation reaction. The reaction condition is to heat and reflux at 83°C for 2 hours. After the reaction is completed, add sodium hydroxide and adjust the pH to neutral. Suction filtration, washing with water three times, the obtained white solid is compound Ⅰ;
[0038] 2) Add 260 g of compound I to 1 L of acetic acid, stir for 30 minutes, and then add sodium nitrite for nitration reaction. The reaction time is 1.5 hours. After the reaction is completed, the solid obtained by suction filtration and drying is compound II;
[0039] 3) Pass hydrogen through 260 g of compound II and 2.6 L of methanol for reduction reaction. The reaction time is 26 hours. After the reaction is completed, the yellow solid obtained by HPLC detection and spin-drying is compound III;
[0040] 4) Under dark and anaerobic conditions, 150g of compound III, 200g of 3-(3,4-dimethoxy-phenolyl)-acrylo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com