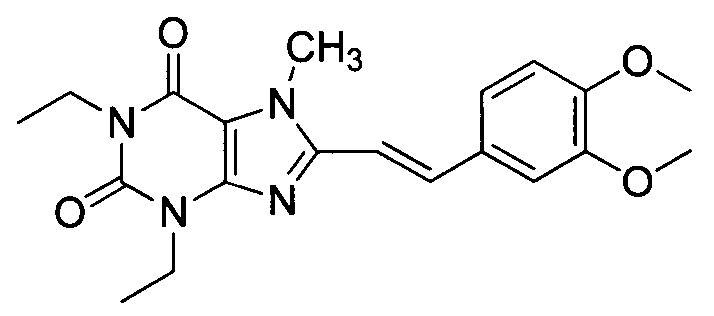
New crystal form of istradefylline and preparation method thereof
A crystal form and crystal technology, applied in the field of medicine, can solve problems such as many adverse reactions, poor tolerance, and reduced curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 Preparation of istradefylline crystal form IV
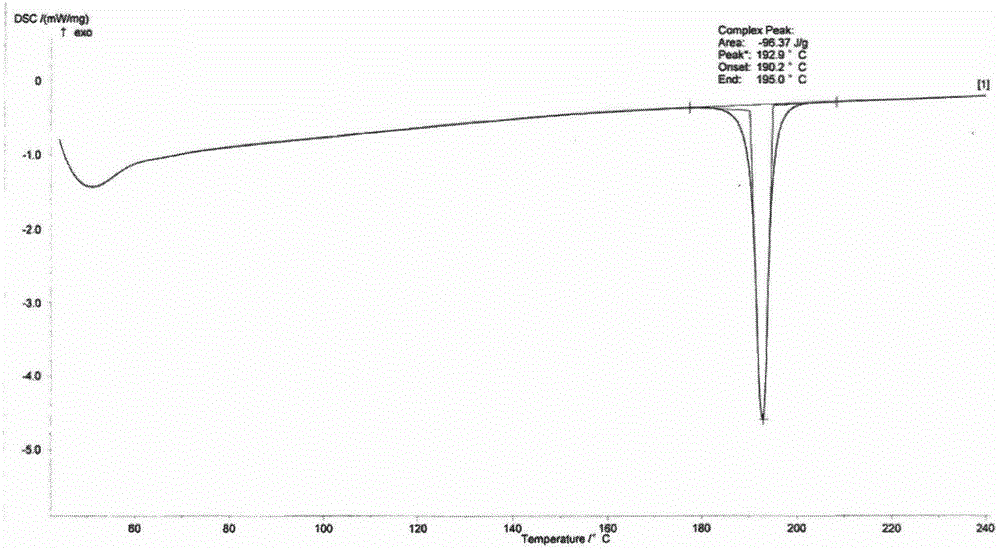
[0021] Weigh 1g of istradefylline into a 50ml single-necked bottle, then add 15ml of n-propanol, stir and heat to 100°C, continue stirring for 3h until reflux occurs, stop heating, cool to room temperature while stirring and crystallizing. The resulting suspension was suction-filtered, the filter cake was washed with 10 ml of n-propanol, and dried at 50°C to constant weight to obtain 0.89g of istradefylline Form IV, with a melting point of 192.8°C as determined by DSC.
Embodiment 2
[0022] Embodiment 2 Preparation of istradefylline crystal form IV
[0023] Weigh 1g of istradefylline into a 50ml single-necked bottle, then add 25ml of n-propanol, stir and heat to 110°C, continue stirring for 6h until reflux occurs, stop heating, cool to room temperature while stirring and crystallizing.
[0024] The obtained suspension was suction-filtered, the filter cake was washed with 10 ml of n-propanol, and dried at 50°C to constant weight to obtain 0.85 g of istradefylline Form IV, with a melting point of 192.9°C as determined by DSC.
Embodiment 3
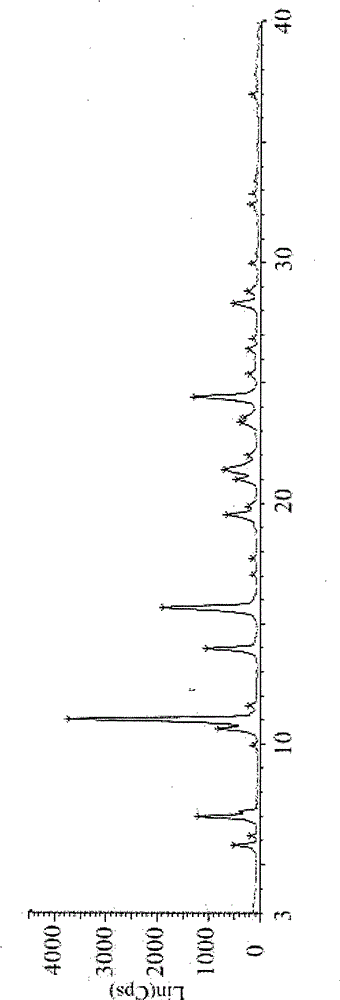
[0025] Example 3 Stability study of istradefylline crystal form IV
[0026] Stability studies have been carried out on istradefylline crystal form IV: [high temperature (60°C), high humidity (90%±5%), light (45001X)], accelerated test (temperature 40±2°C, relative humidity 75% ±5%) and long-term test (temperature 25±2°C, relative humidity 60%±10%)] and carry out X-ray powder diffraction test on the sample after grinding and tableting.
[0027] Table 1 Results of determination of stability of crystal form IV
[0028]
[0029] Table 2 Crystal Form IV Influencing Factor Test Determination Results
[0030]
[0031]
[0032] Table 3 Form IV Stability Test Determination Results
[0033]
[0034] The test results show that: after grinding and tableting, the X-ray diffraction test shows that the crystal form data of this product has not changed significantly, indicating that this product has good stability during the preparation process. In the test of influencing facto...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com