Improved synthesis technology of istradefylline
A technology of istradefylline and a process method, which is applied in the research field of the synthesis process route of istraphylline, can solve the problems of increasing the difficulty of purifying intermediates and products, high price, etc. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
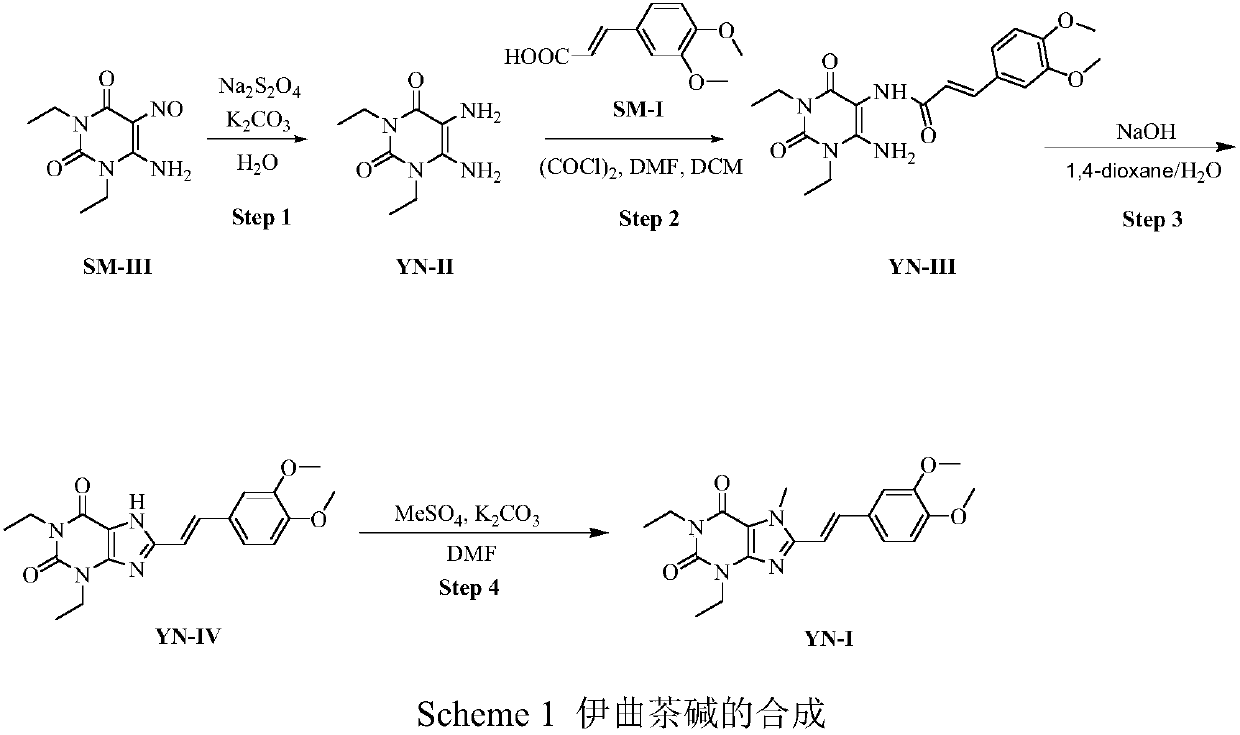
[0014] Add 650g (3.06mol) of the starting material SM-III into a 20L reaction flask, add 10L of 30% aqueous potassium carbonate solution to it, stir at 30-50°C, add 2964g (15.3mol) of 90% hydrosulfite to the reaction solution , stirred at room temperature for 1.0 hour, the solution was clear, TLC (DCM:MeOH=50:1) showed that the starting material disappeared, and the aqueous solution of intermediate YN-II was obtained, which was directly used in the next reaction without treatment.
[0015] Synthesis of YN-III
[0016] We replace the more corrosive thionyl chloride with oxalyl chloride, which can improve the yield of intermediate YN-III; if the reaction solvent is replaced by tetrahydrofuran or dioxane from dichloromethane, the reaction yield is significantly reduced, Therefore choose to adopt dichloromethane as condensation reaction solvent. The feed of the starting material (E)-3,4-dimethoxyphenylacrylic acid (SM-I) was reduced to 0.95 equivalents. The result has no differe...
Embodiment 2
[0018] Add 605.2g (2.91mol) of the starting material SM-I into a 10L reaction flask, add 3.5L of dichloromethane and 6.5mL of DMF, stir at 25°C, and drop 369.4g (2.91mol) of oxalyl chloride within 20 minutes. After stirring at 25°C for 1.5 hours, the solution became clear. The reaction solution was slowly added dropwise to the aqueous solution of YN-II obtained in step 1, and the temperature of the reaction solution was controlled at 0-5°C. After the drop was completed, stir at room temperature for 1 hour, and monitor by TLC (DCM:MeOH=50:1, UV 254nm) that the reaction of YN-II was complete. A light yellow solid was precipitated, filtered, recrystallized from 50% ethanol, and the obtained light yellow solid was dried overnight at 60°C to obtain 1016g of intermediate YN-III with a yield of 90%.
[0019] Synthesis of YN-IV
[0020] In this step of synthesis, the intermediate YN-III was treated with 10M sodium hydroxide, and the reaction was complete in 1h, and many impurities w...
Embodiment 3
[0022] Take YN-III 895.1g (2.30mol) into a 20L reaction flask, add 5.75L each of dioxane and 5M NaOH aqueous solution, stir at 60°C for 2 hours, monitor by TLC (DCM:MeOH=30:1, UV 254nm) basically no raw material remains. After cooling down to room temperature, 2657 ml of concentrated hydrochloric acid was added thereto to adjust the pH to 2, and solids were precipitated. Stop heating, naturally cool down to room temperature, stir for 3 hours, and filter. 3.16 L of water was washed twice to obtain a white solid, which was dried at 45°C for 17 hours to obtain 683.6 g of intermediate YN-IV with a yield of 80.1%.
[0023] Intermediate YN-IV quality control method:
[0024] Synthesis of Crude Itradefylline (YN-I)
[0025] We investigated the reaction solvent, the feed ratio of methyl iodide and potassium carbonate, etc., and used TLC or HPLC to monitor the reaction as required. After experimenting, it was determined that the intermediate YN-IV was used as the raw material, pota...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com