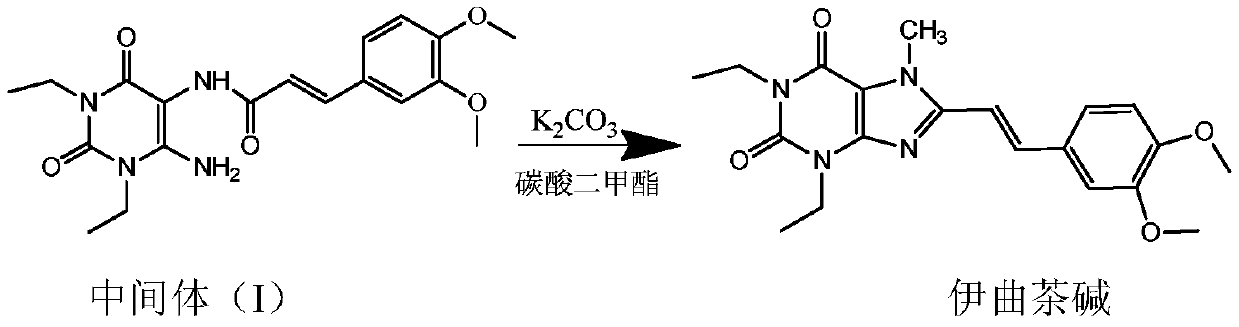
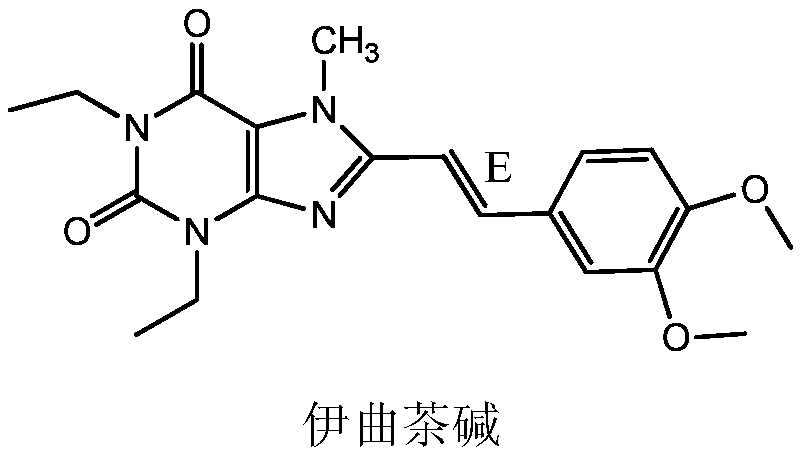
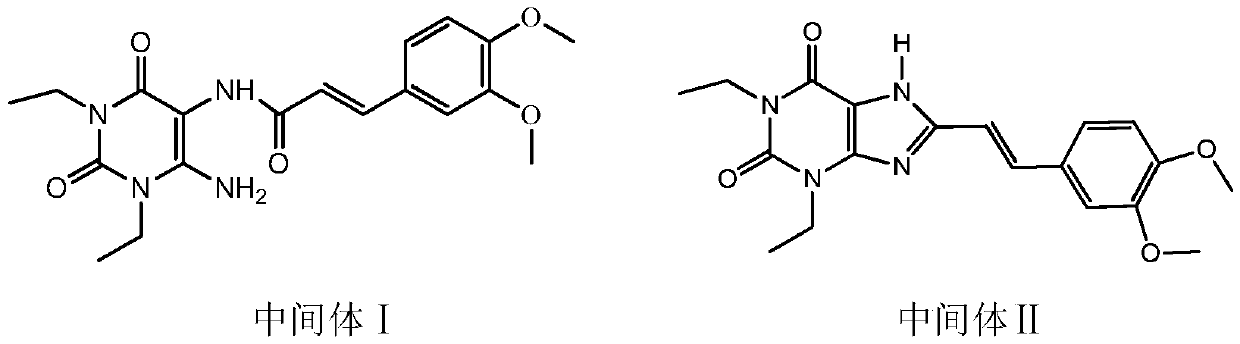
Istradefylline preparation method
A technology of istradefylline and reaction is applied in the new field of istradefylline preparation, which can solve the problems of high toxicity of methyl iodide, complicated feeding process and the like, and achieve the effects of saving organic solvent, high reaction conversion rate and reducing production amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] In a clean and dry reaction flask, put 15.8g (0.04mol) Intermediate I, 68ml DMF, 7.8g (0.056mol) potassium carbonate, raise the temperature to 120°C, keep the reaction temperature for 30 minutes, and then add 15.6g (0.17mol) dicarbonate to it For the methyl ester, the temperature is controlled between 120°C and 140°C for 2 hours. The temperature of the reaction solution was lowered to 0-5°C, and the temperature was kept for 1 hour to crystallize. After filtration, istraphylline was obtained as light yellow needle-like crystals, 8.2 g, with a purity of 99% and a yield of 52.4%.
Embodiment 2
[0032] In a clean and dry reaction flask, put 15.8g (0.04mol) of Intermediate I, 68ml of N-methylpyrrolidone, 7.8g (0.15mol) of potassium carbonate, raise the temperature to 150°C, keep the reaction temperature for 30 minutes, and then add 5.4g ( 0.06mol) dimethyl carbonate, the temperature is controlled at 150°C for 2 hours. The temperature of the reaction liquid was lowered to 10-20°C, and the temperature was kept for 1 hour to crystallize. After filtration, itraphylline was obtained as light yellow needle-like crystals, 7.1 g, with a purity of 99% and a yield of 45.4%.
Embodiment 3
[0034] In a clean and dry reaction flask, put 15.8g (0.04mol) Intermediate I, 68ml DMF, 7.8g (0.15mol) potassium carbonate, raise the temperature to 100°C, keep the reaction temperature for 30 minutes, and then add 36.0g (0.4mol) dicarbonate to it Methyl ester, control the temperature at 100°C to react for 2 hours. The temperature of the reaction liquid was lowered to 5-10°C, and the temperature was kept for 1 hour to crystallize. After filtration, itraphylline was obtained as light yellow needle-like crystals, 7.3 g, with a purity of 99% and a yield of 46.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com