A kind of preparation method of istradefylline
A technology of istradefylline and its compound, which is applied in the field of preparation of istradefylline, can solve problems such as ozone voids, poor atom economy, and large equipment corrosion, and achieve the goals of improving stability, increasing total yield, and reducing costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
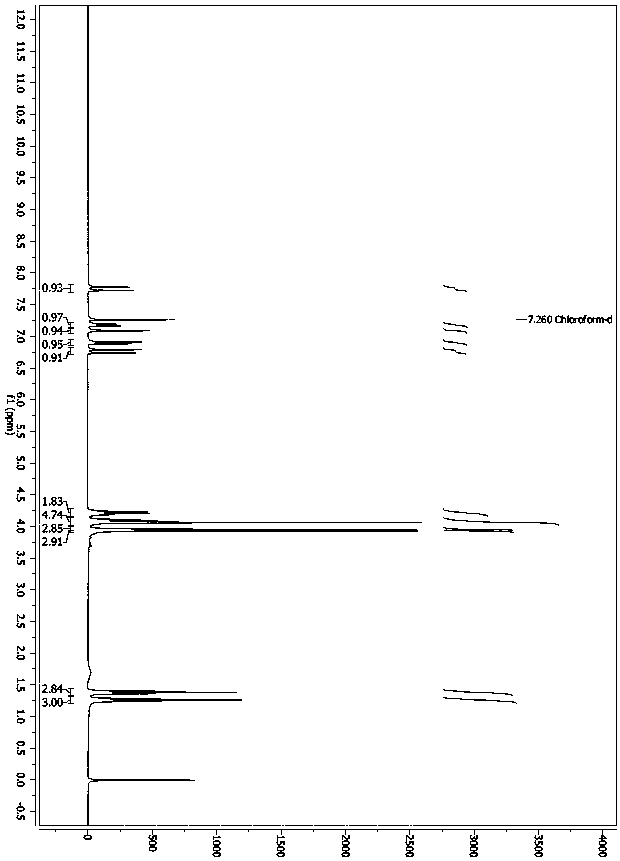
Image
Examples
Embodiment 1
[0031] Preparation of Compound III
[0032] Under stirring, put 6-amino-1,3-diethyl-5-nitroso-1 into the reaction kettle in sequence H -Pyrimidine-2,4-dione (compound II) (140.0 g, 0.66 mol), 1100 ml of methanol and 20.0 g of 10% Pd / C, control the speed at 100 r / min, and control the temperature at 15°C. Hydrogen pressurized (0.50 MPa-0.60 MPa) reaction, TLC monitoring reaction is complete. The Pd / C was removed by filtration and washed with methanol. After concentration, 2 mol / L hydrochloric acid ethanol solution was added dropwise. After dropping, stir and crystallize below 10°C. Filter and wash the filter cake with absolute ethanol. The filter cake was collected and air-dried at 60°C to obtain 130.1 g of off-white powder (Compound III), with a yield of 84%.
[0033] Preparation of Compound IV
[0034] Under stirring, add 500 ml of dichloromethane, 5,6-diamino-1,3-diethyl-1 H - Pyrimidine-2,4-dione hydrochloride (compound III) (120.0 g, 0.51 mol) and acetyl chloride (44...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com