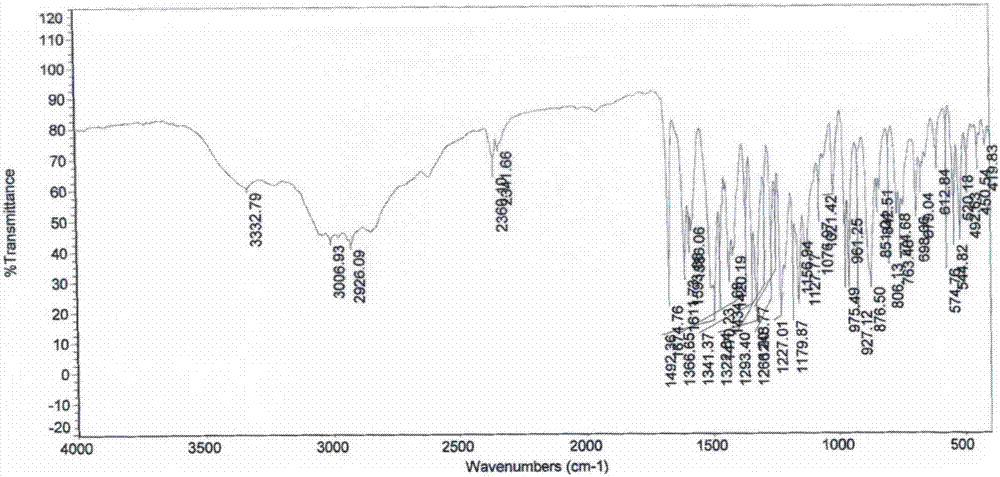
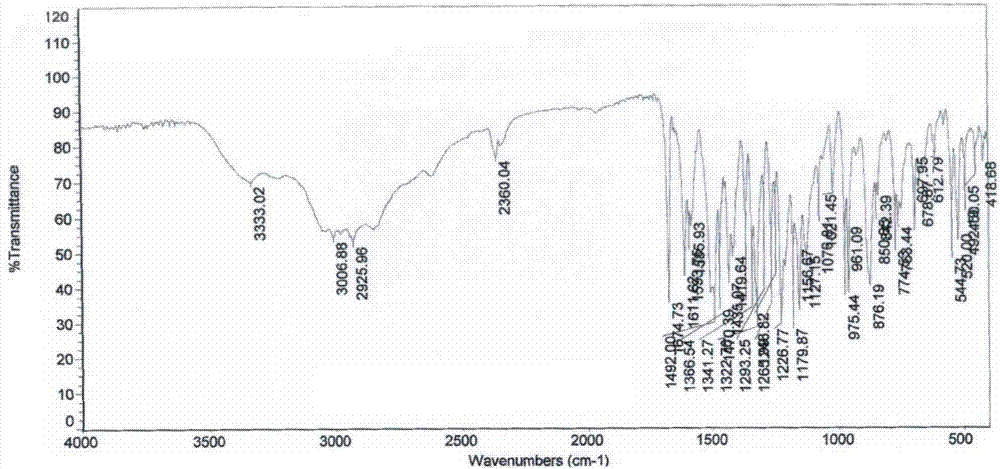
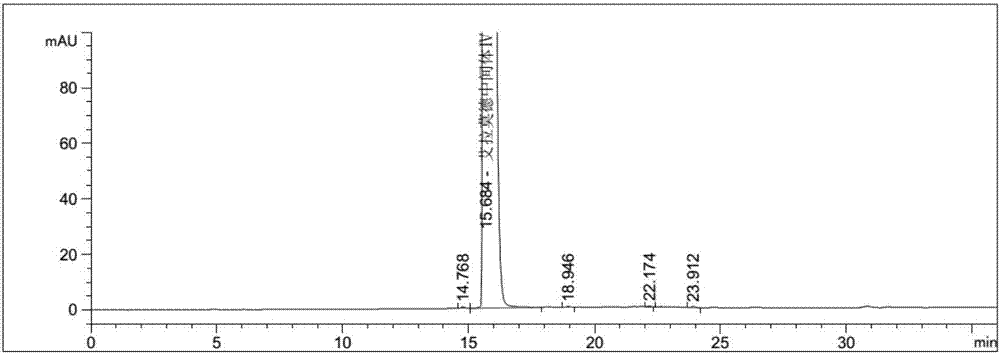
Preparation method of iguratimod intermediate IV
An intermediate and weight ratio technology, which is applied in the field of medicine and chemical industry, can solve the problems of great harm to the human body, difficulty in mixing, and large environmental pollution, and achieve the effects of improving product purity, facilitating post-processing, and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] After adding 300mL of nitromethane to the 1000mL reaction flask, add 203g of anhydrous aluminum trichloride in batches, and add 114g of aminoacetonitrile hydrochloride after cooling to room temperature; when the temperature drops below 20℃, add 150g of intermediate III(4 -Phenoxy-3-methanesulfonamido anisole); After stirring for 10 minutes, dry hydrogen chloride gas is introduced, and the reaction is completed by airing for about 15 hours. Slowly add the above reaction solution to 1200g 2mol / L dilute hydrochloric acid, control the temperature below 10℃, stir for 1h after adding, filter, wash the solid with a small amount of water (about 80mL), drain; then use 150mL ethyl acetate Beating, filtering, draining, and drying; then the solid is refluxed with twice the volume of 95% (volume percentage) ethanol for 1 hour to cool, filtered, and the solid is dried until the moisture content is qualified to obtain the final product (α-amino-2-methoxy- 4-Methanesulfonamido-5-phenoxy...
Embodiment 2
[0028] After adding 300mL of nitromethane to the 1000mL reaction flask, add 203g of anhydrous aluminum trichloride in batches, and add 114g of aminoacetonitrile hydrochloride after cooling to room temperature; when the temperature drops below 20℃, add 150g of intermediate III(4 -Phenoxy-3-methanesulfonamido anisole); After stirring for 10 minutes, dry hydrogen chloride gas is introduced, and the reaction is completed by airing for about 15 hours. Slowly add the above reaction solution to 1200g 1mol / L dilute hydrochloric acid, control the temperature below 10℃, stir for 1h after adding, filter, wash the solid with a small amount of water (about 80mL), drain; then use 150mL ethyl acetate Beating, filtering, draining, and drying; then the solid is refluxed with twice the volume of 95% (volume percentage) ethanol for 1 hour to cool, filtered, and the solid is dried until the moisture content is qualified to obtain the final product (α-amino-2-methoxy- 4-Methanesulfonamido-5-phenoxy...
Embodiment 3
[0030] After adding 300mL of nitromethane to the 1000mL reaction flask, add 203g of anhydrous aluminum trichloride in batches, and add 114g of aminoacetonitrile hydrochloride after cooling to room temperature; when the temperature drops below 20℃, add 150g of intermediate III(4 -Phenoxy-3-methanesulfonamido anisole); After stirring for 10 minutes, dry hydrogen chloride gas is introduced, and the reaction is completed by airing for about 15 hours. Slowly add the above reaction solution to 1200g 4mol / L dilute hydrochloric acid, control the temperature below 10℃, stir for 1h after adding, filter, wash the solid with a small amount of water (about 80mL), drain; then use 150mL ethyl acetate Beating, filtering, draining, and drying; then the solid is refluxed with twice the volume of 95% (volume percentage) ethanol for 1 hour to cool, filtered, and the solid is dried until the moisture content is qualified to obtain the final product (α-amino-2-methoxy- 4-Methanesulfonamido-5-phenoxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com