Method for preparing high-yield and high-purity Iguratimod intermediate
An intermediate, methanesulfonamide technology, applied in the field of pharmaceutical preparation, can solve the problems of volatile, incomplete reaction, no purification steps, etc., and achieve the effects of easy industrial production, reduced environmental hazards, and good sample stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
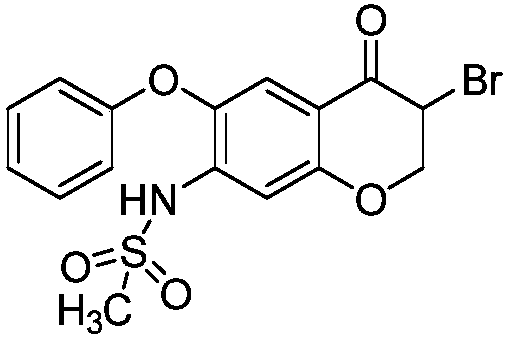
[0037] Example 1a (preparation of 3-bromo-7-methanesulfonamide-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one crude product)
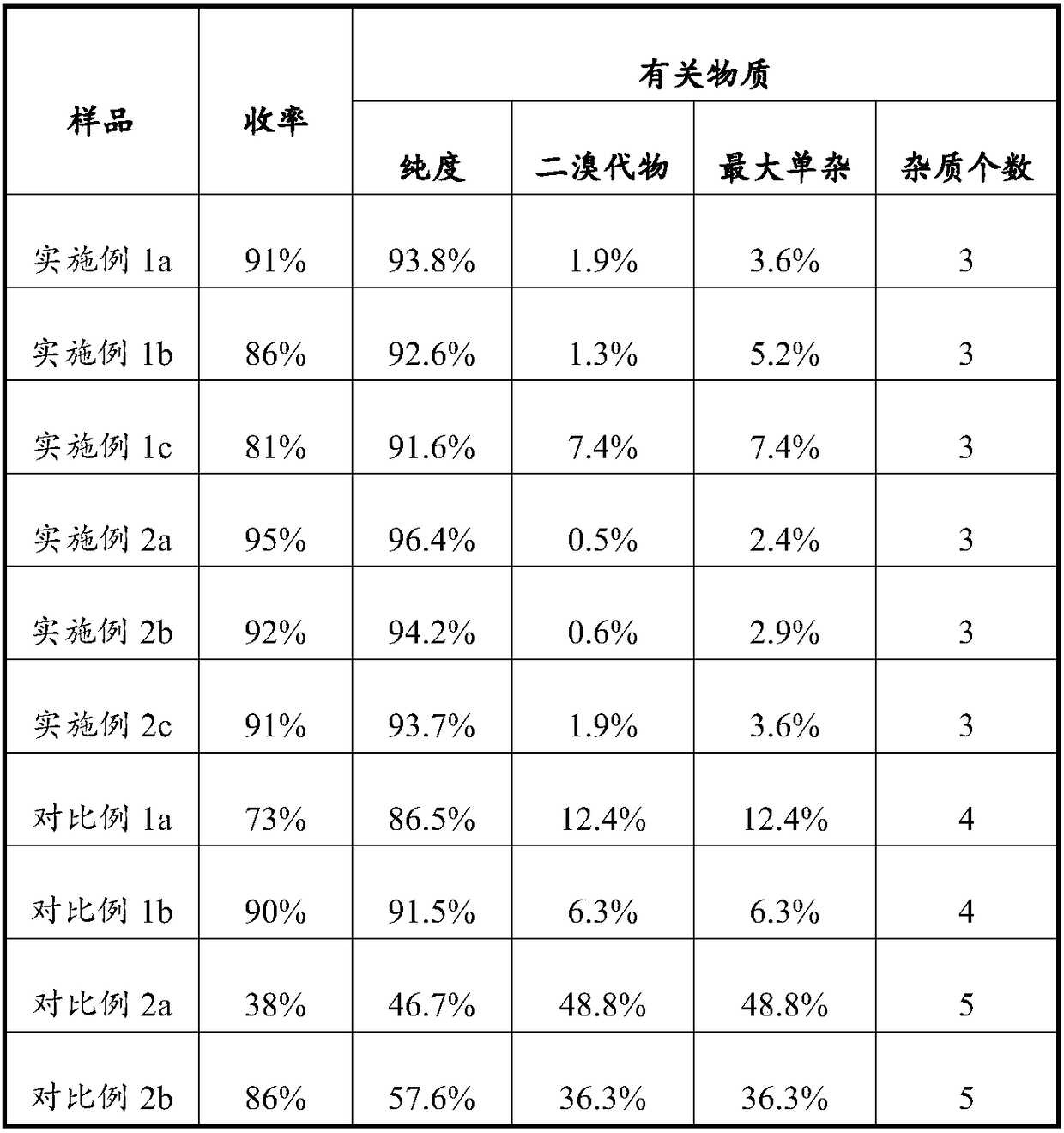
[0038]To a 50L dry reactor, add 4.5kg ethyl acetate, 3.15kg methanol, 2.1kg copper bromide, stir for 30 minutes, then add 1kg 7-methanesulfonamide-6-phenoxy-4H-1-benzo - The mixture of 2,3-dihydropyran-4-one and 20kg dichloromethane, after the addition is completed, heat up to 25-35°C and stir for 3-4 hours, filter, and the filtrate uses the mass percentage of sodium thiosulfate Wash three times with 2% sodium thiosulfate aqueous solution, 10kg each time, collect the organic phase; then concentrate the organic phase under reduced pressure at 30-60°C to obtain 1.13kg of 3-bromo-7-methanesulfonamide-6-phenoxy The crude product of yl-4H-1-benzo-2,3-dihydropyran-4-one has a yield of 91%, and a purity of 93.8% by HPLC.
Embodiment 1
[0039] Example 1b (preparation of 3-bromo-7-methanesulfonamide-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one crude product)
[0040] To a 50L dry reactor, add 4.5kg ethyl acetate, 3.15kg ethanol, 2.1kg copper bromide, stir for 30 minutes, then add 1kg 7-methanesulfonamide-6-phenoxy-4H-1-benzo - The mixture of 2,3-dihydropyran-4-one and 20kg dichloromethane, after the addition is completed, heat up to 25-35°C and stir for 3-4 hours, filter, and the filtrate uses the mass percentage of sodium thiosulfate Wash three times with 2% sodium thiosulfate aqueous solution, 10kg each time, collect the organic phase; then concentrate the organic phase under reduced pressure at 30-60°C to obtain 1.06kg of 3-bromo-7-methanesulfonamide-6-phenoxy The crude product of -4H-1-benzo-2,3-dihydropyran-4-one has a yield of 86%, and a purity of 92.6% by HPLC.
[0041] Example 1c (preparation of 3-bromo-7-methanesulfonamide-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one crude product)
[0042] To a 50L d...
Embodiment 2
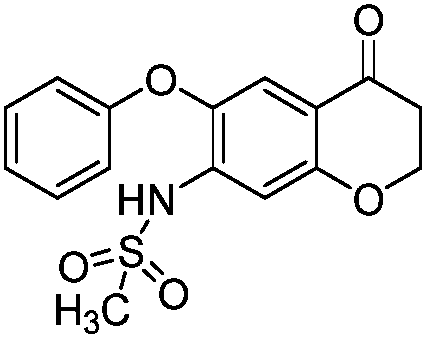
[0043] Example 2a (purification of 3-bromo-7-methanesulfonamide-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one crude product)
[0044] Into a 100L dry reaction kettle, add 40kg of petroleum ether, lower the temperature to 0-10°C, and then dropwise add the 3-bromo-7-methanesulfonamide-6-phenoxy-4H-1-benzene prepared in Example 1a The mixed solution of crude and-2,3-dihydropyran-4-one and 6kg of dichloromethane, after dropping, keep stirring for 4h, filter, wash the filter cake with 1kg of petroleum ether, collect the filter cake, and heat it at 30~40℃ Dry under vacuum, weigh and check the purity to obtain a total of 1.07kg of 3-bromo-7-methanesulfonamide-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one , the yield was 95%, and the HPLC detection purity was 96.4%.
[0045] Example 2b (purification of 3-bromo-7-methanesulfonamide-6-phenoxy-4H-1-benzo-2,3-dihydropyran-4-one crude product)
[0046] Into a 100L dry reactor, add 40kg of petroleum ether, lower the temperature to 0-10°C, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com