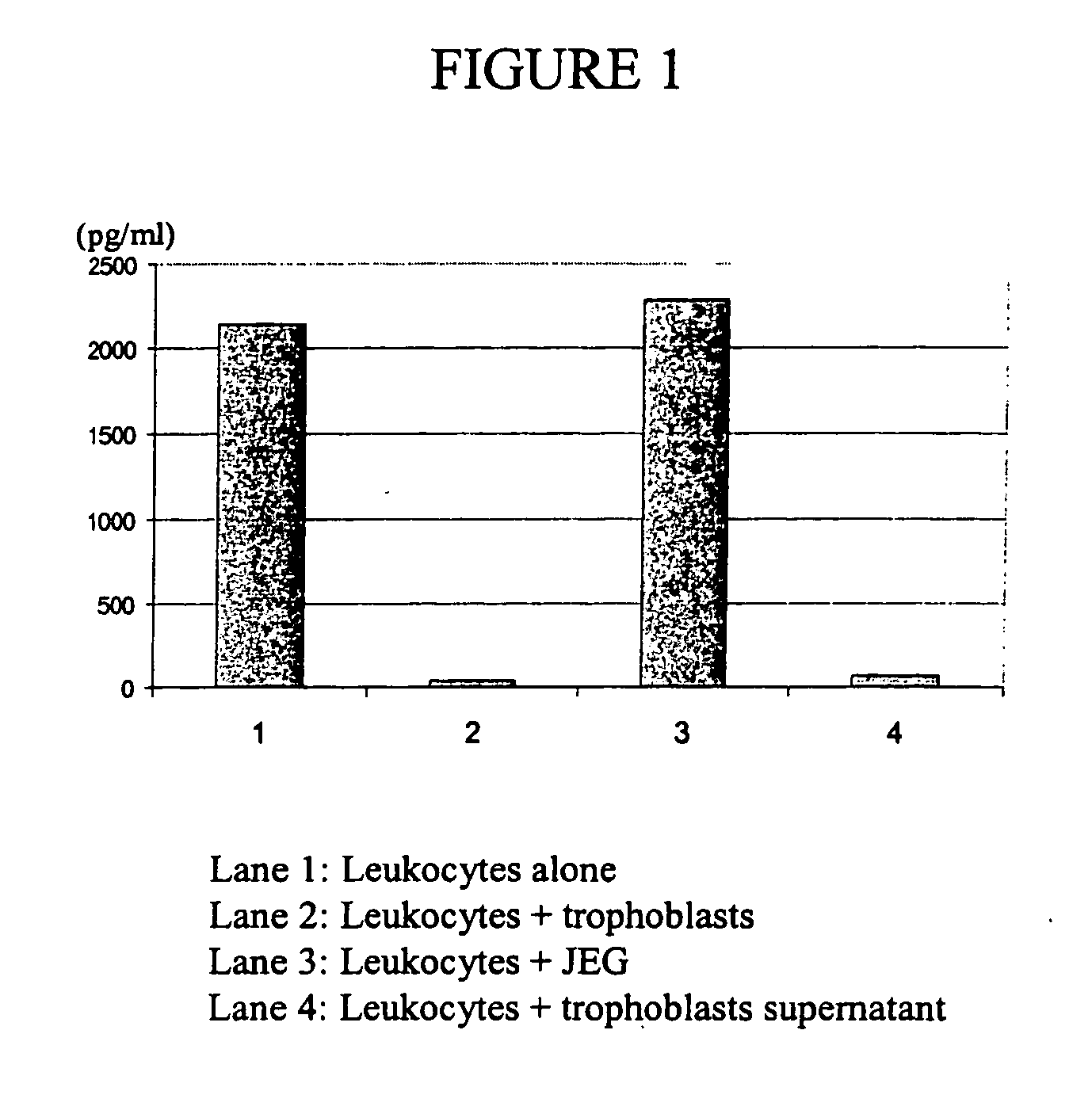
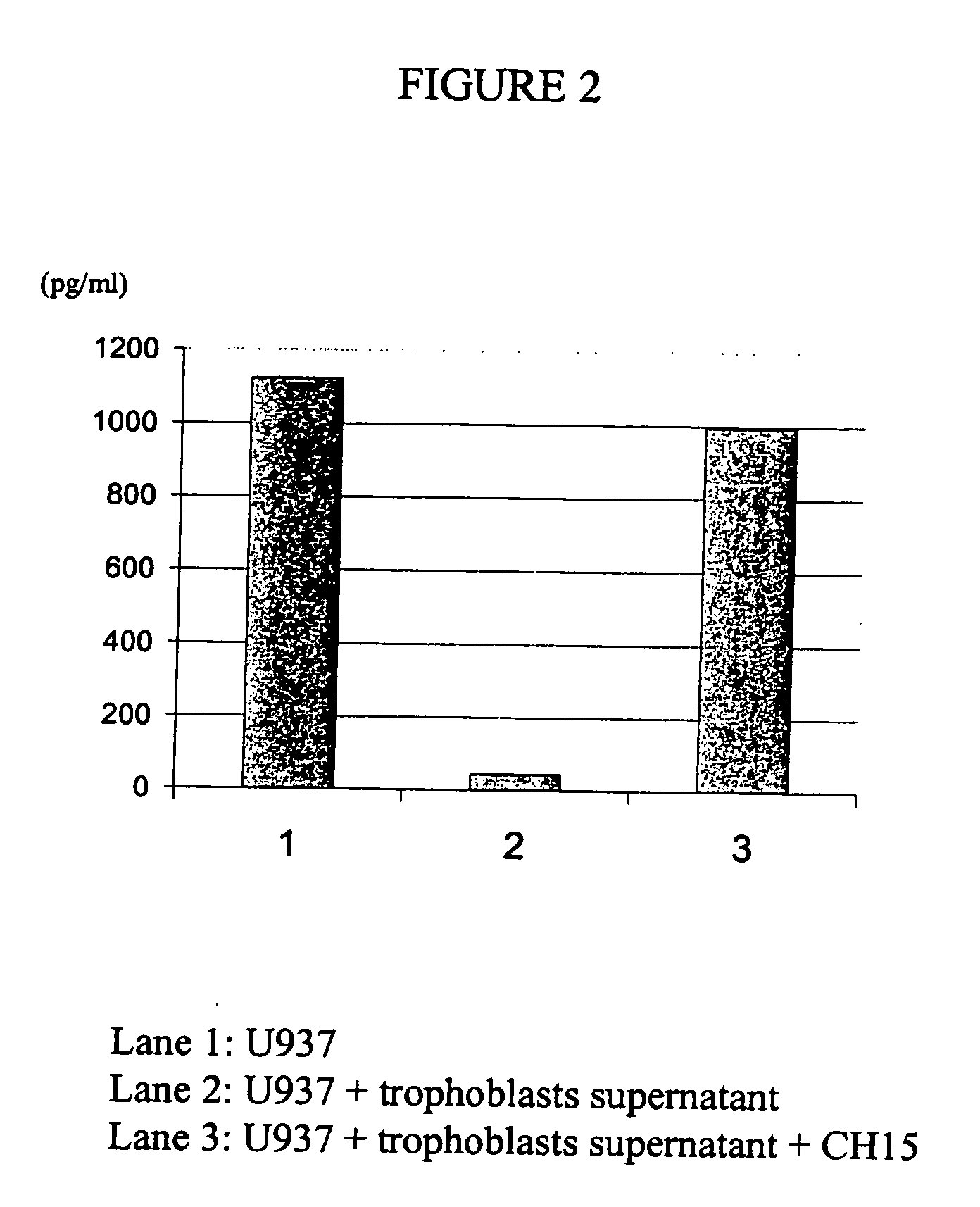
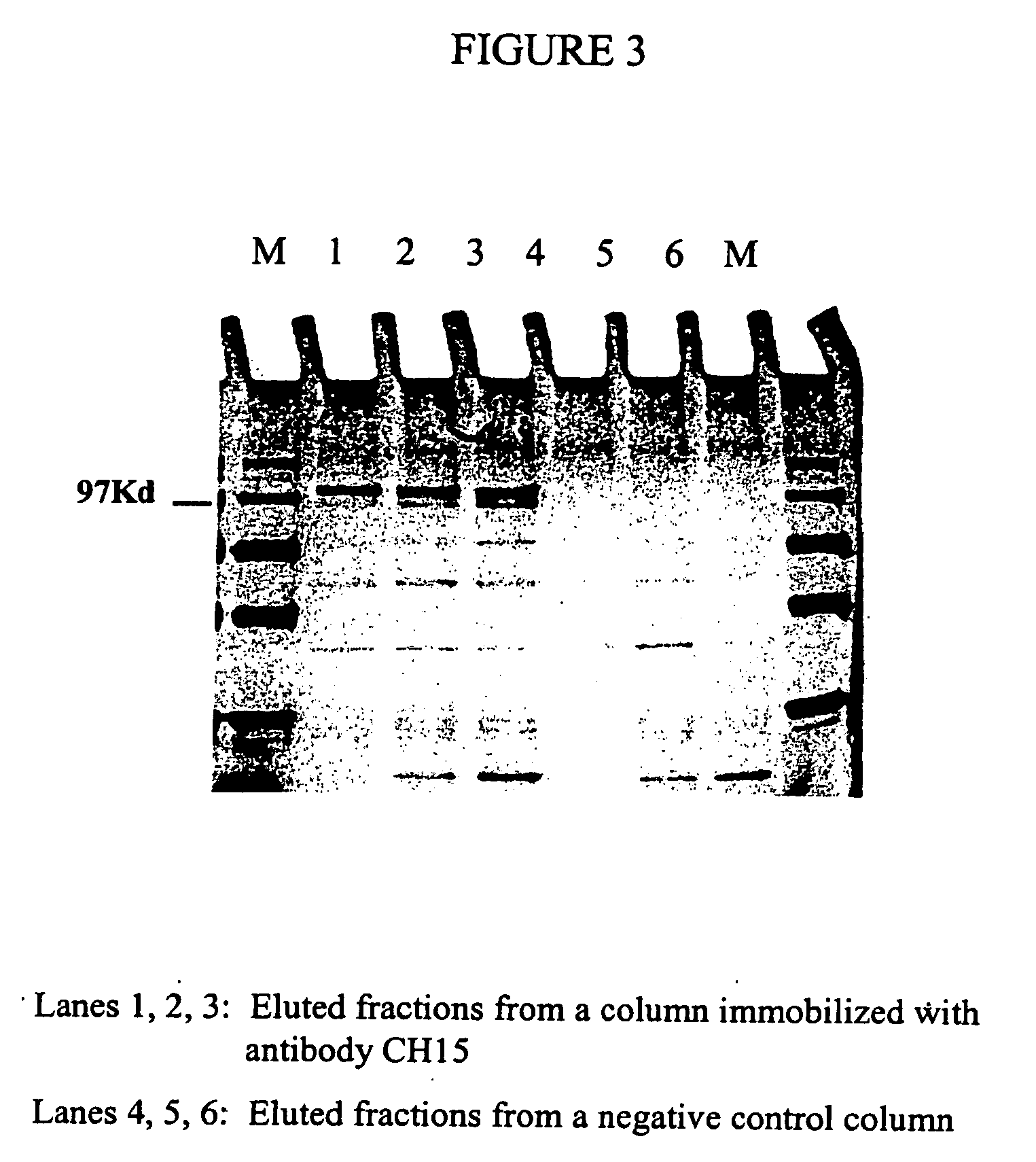
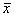Patents
Literature
88results about How to "Stop progress" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
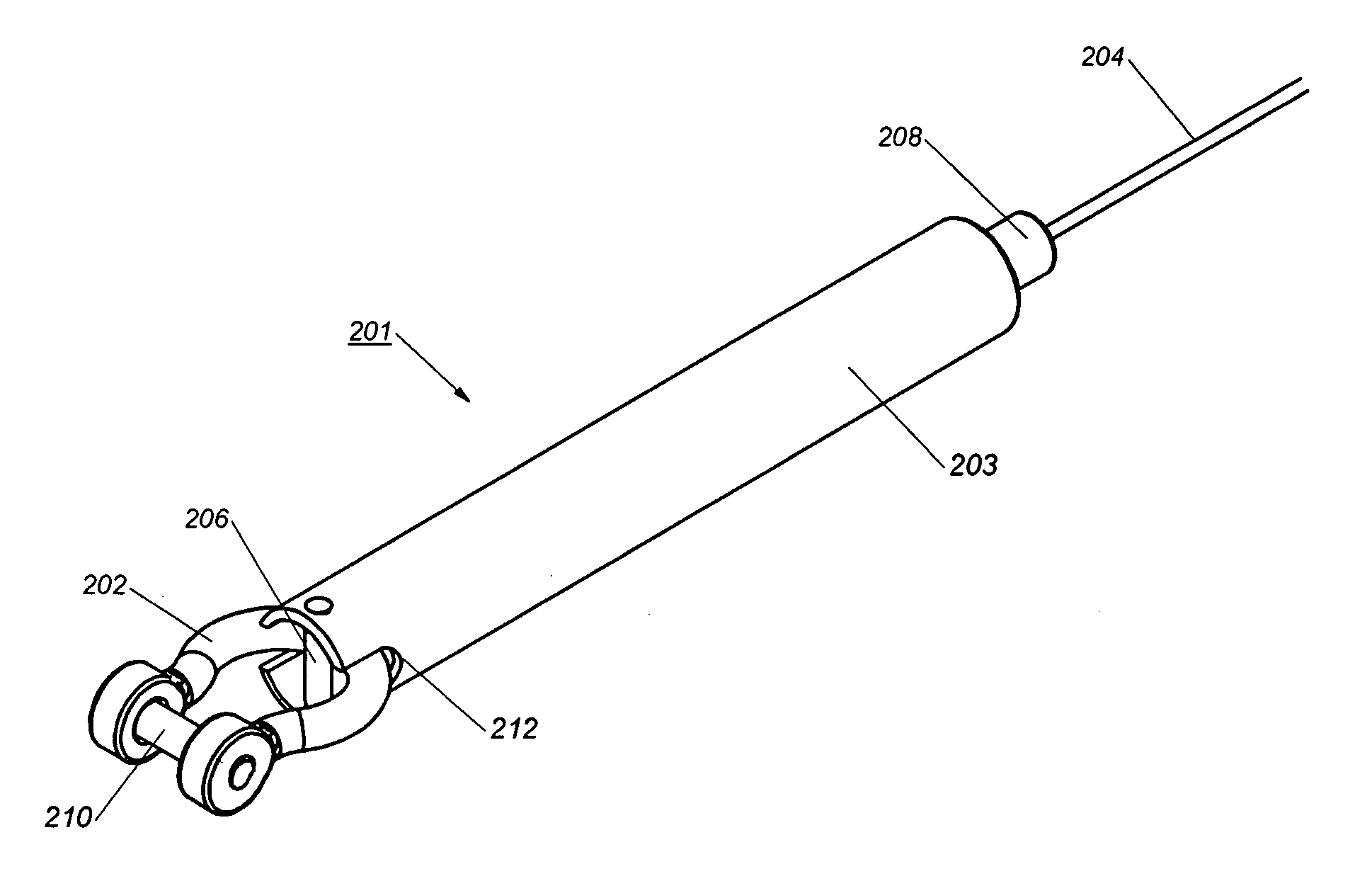
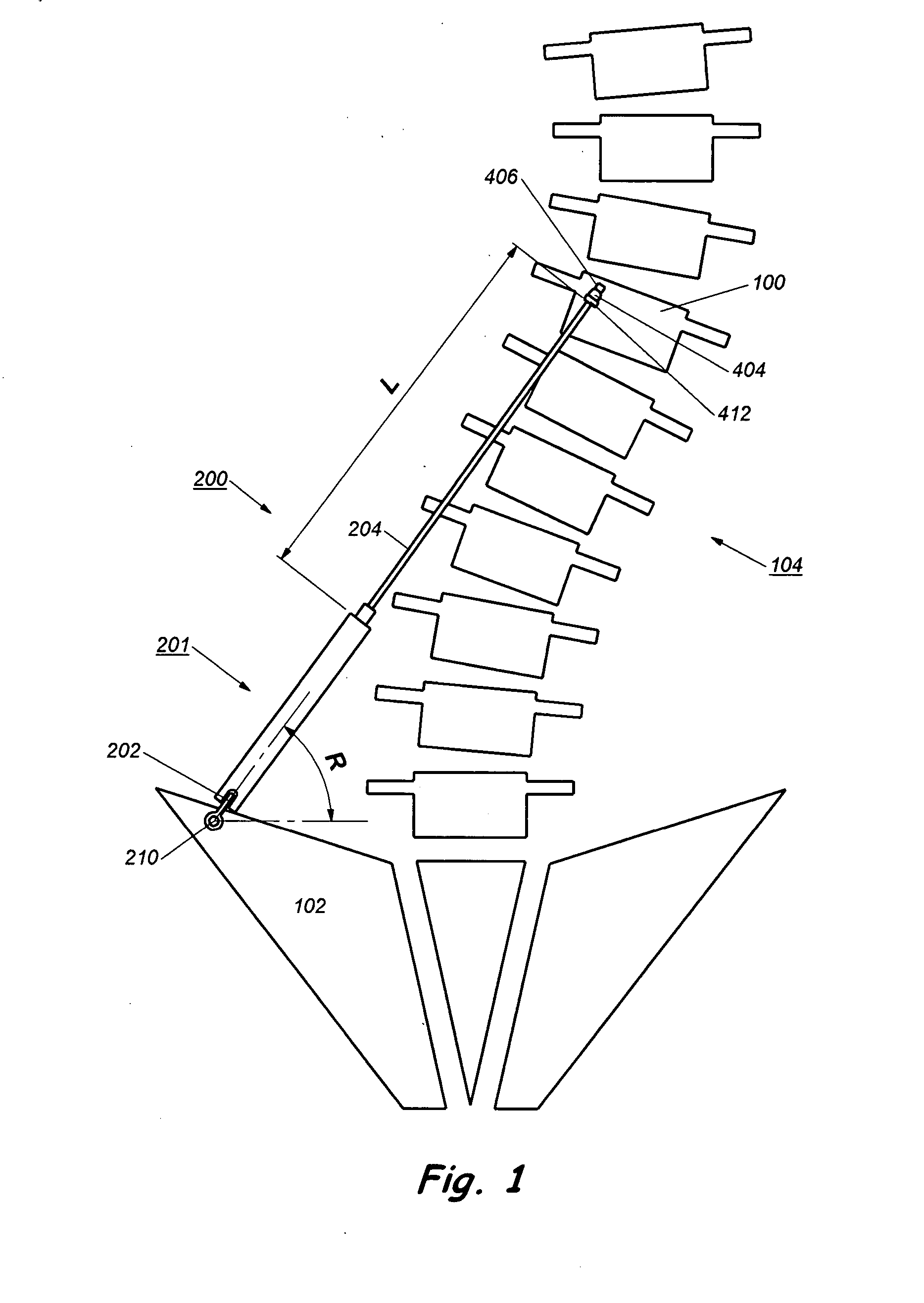
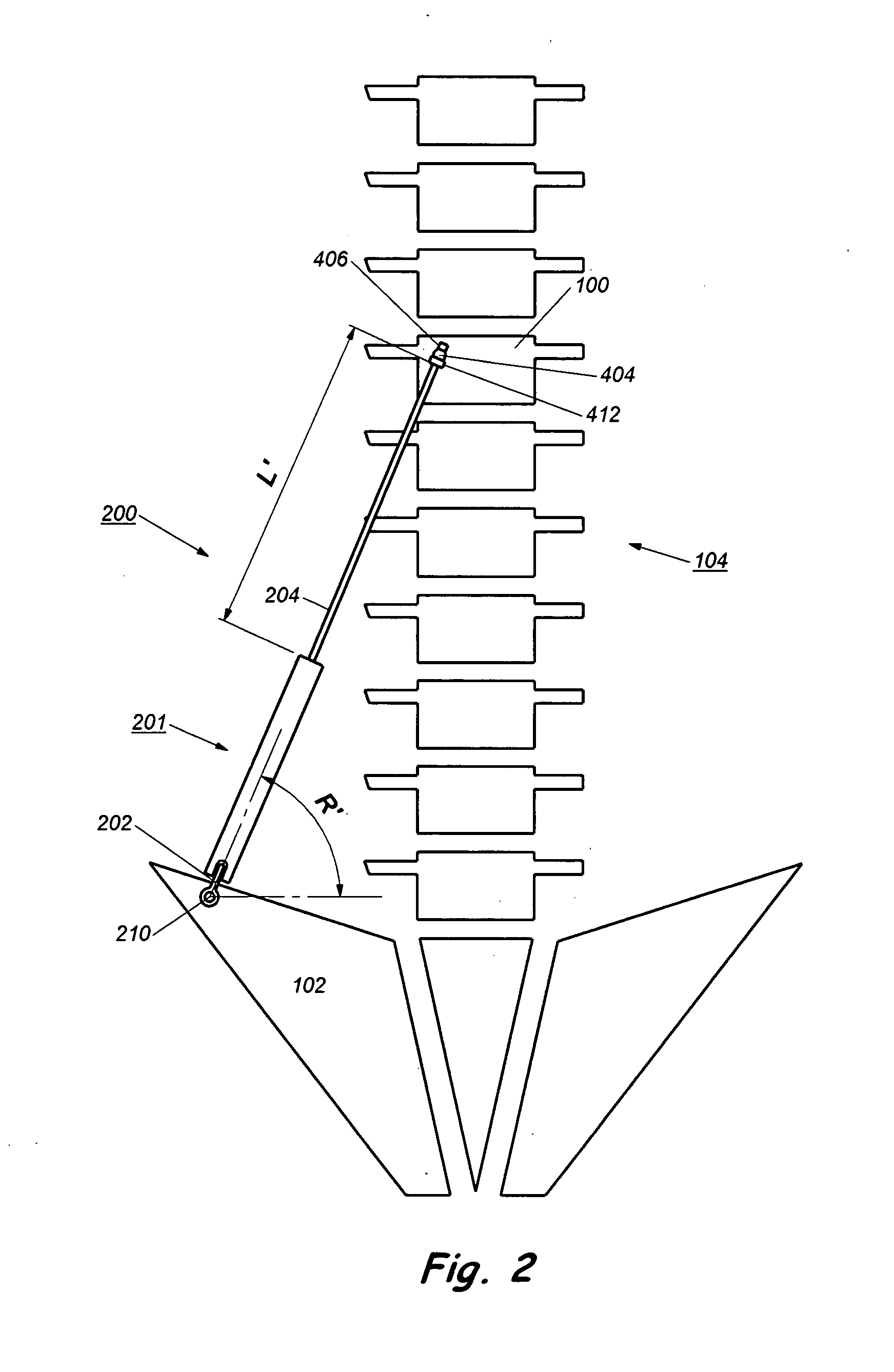
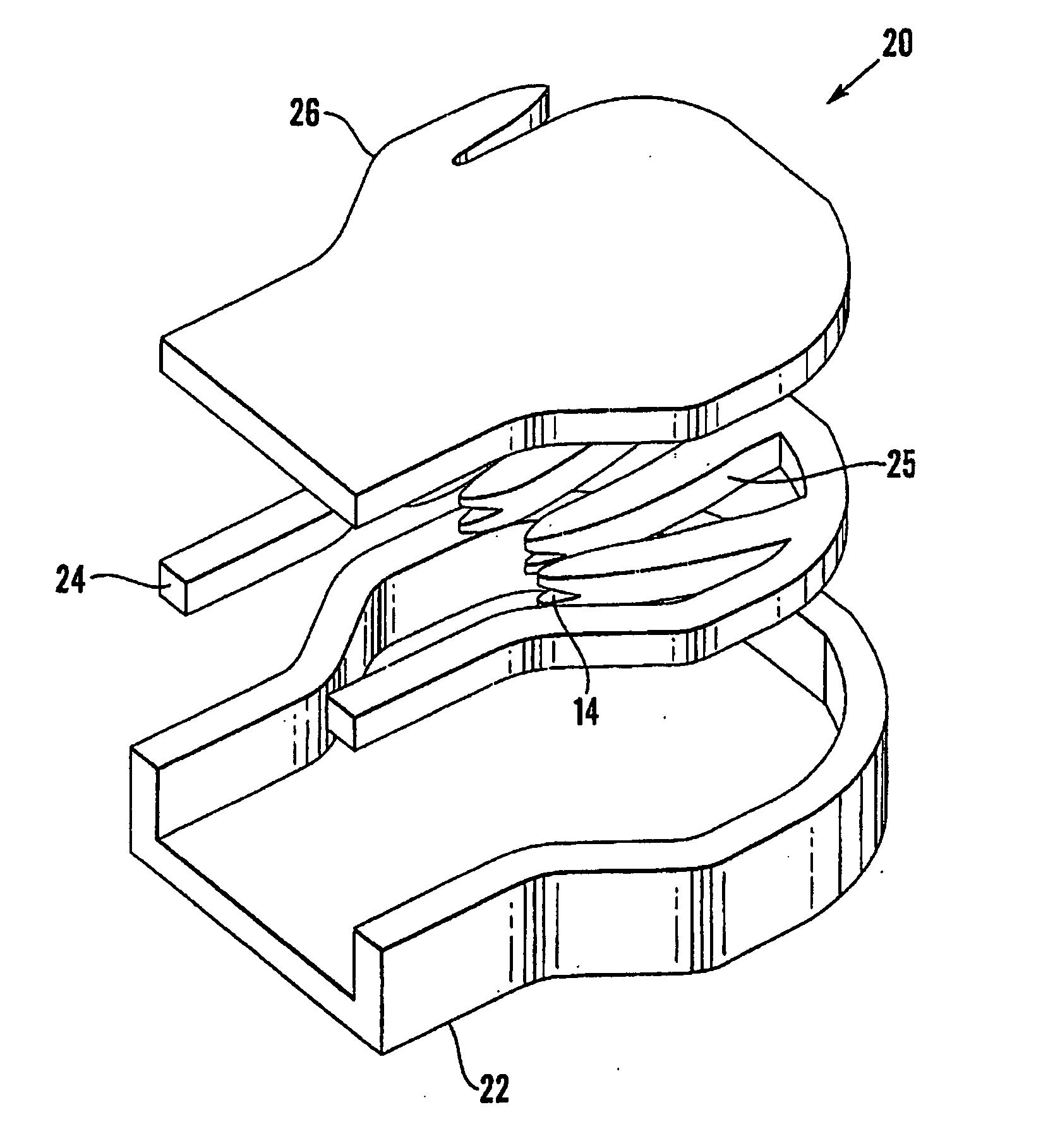
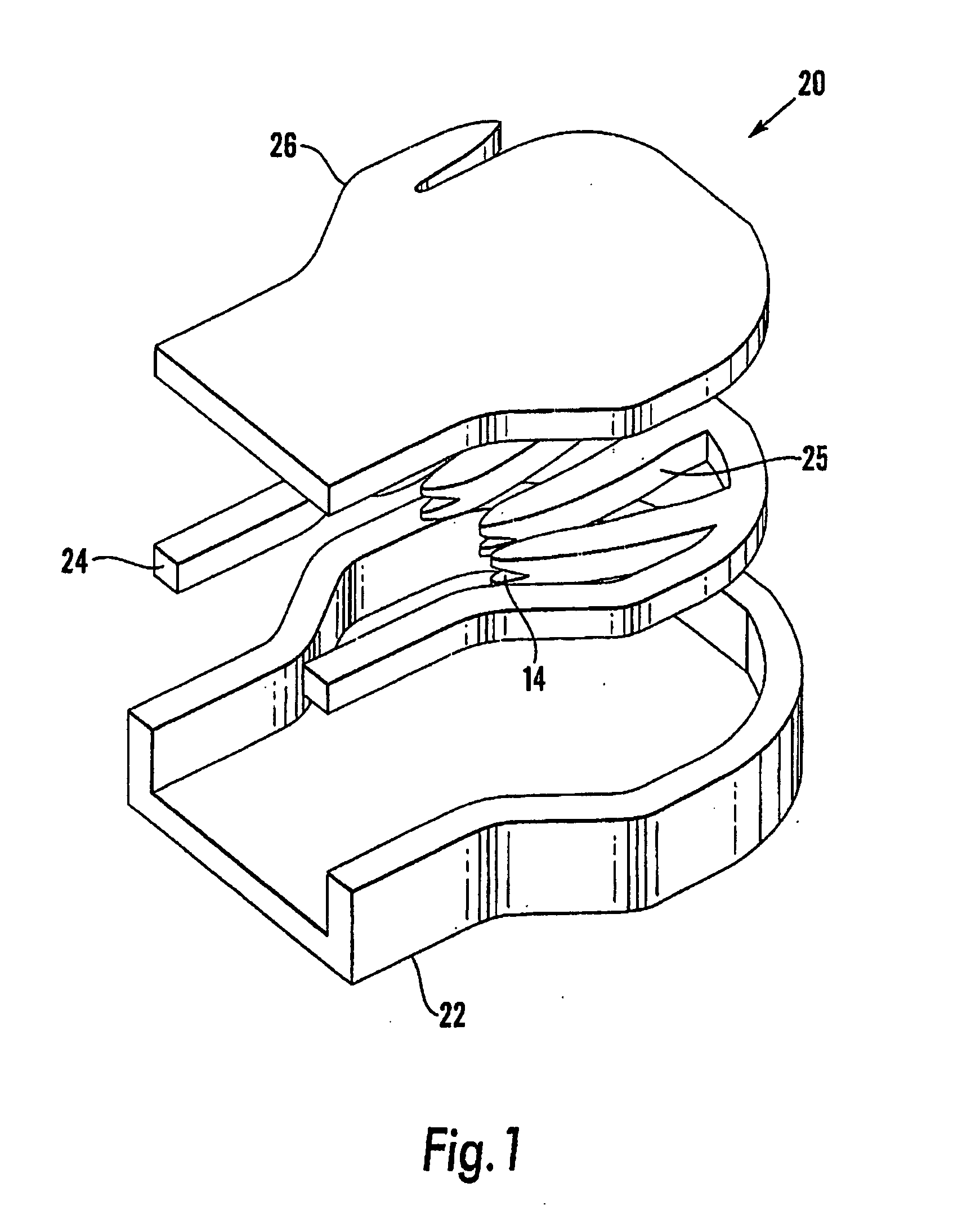
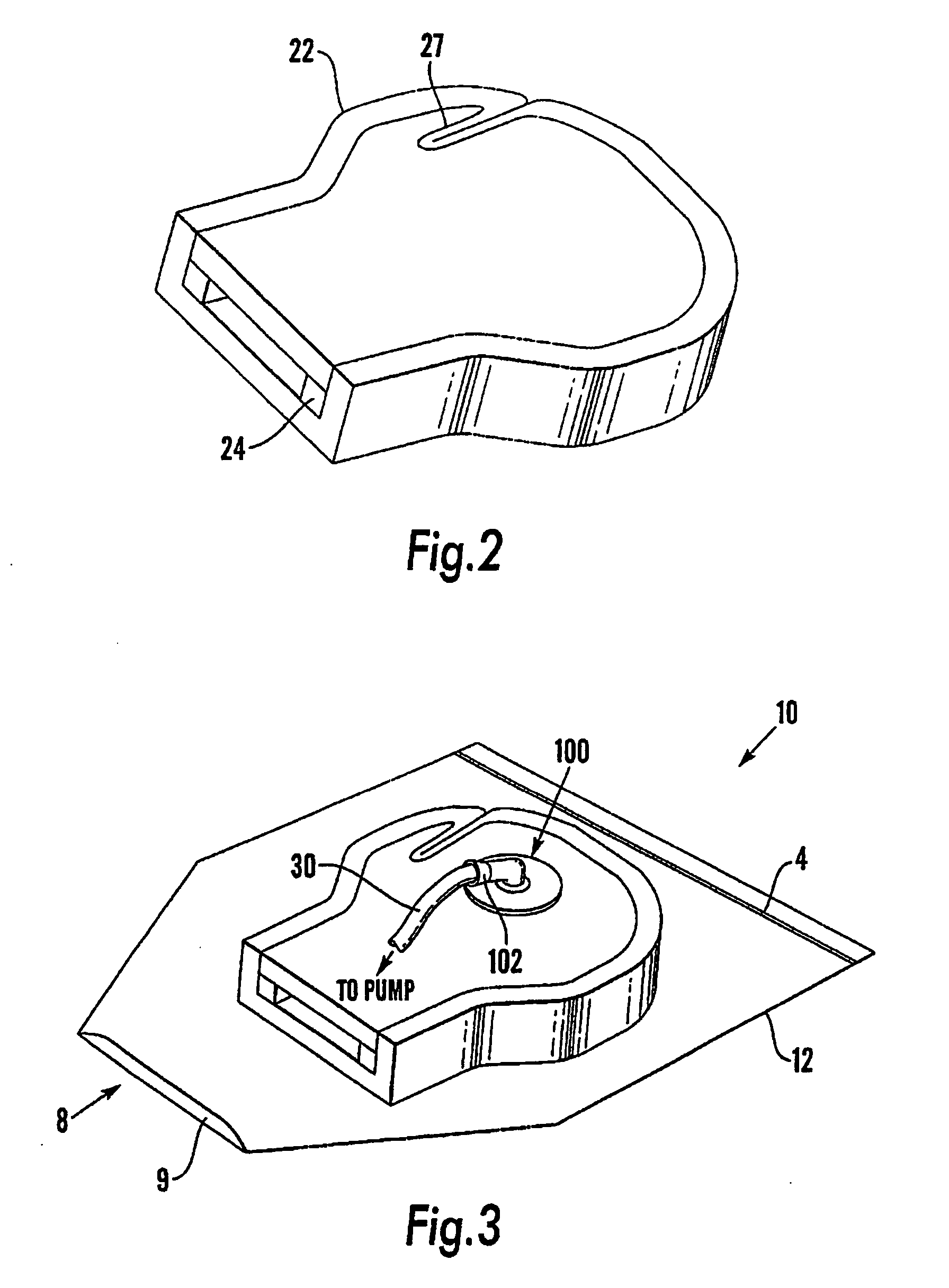
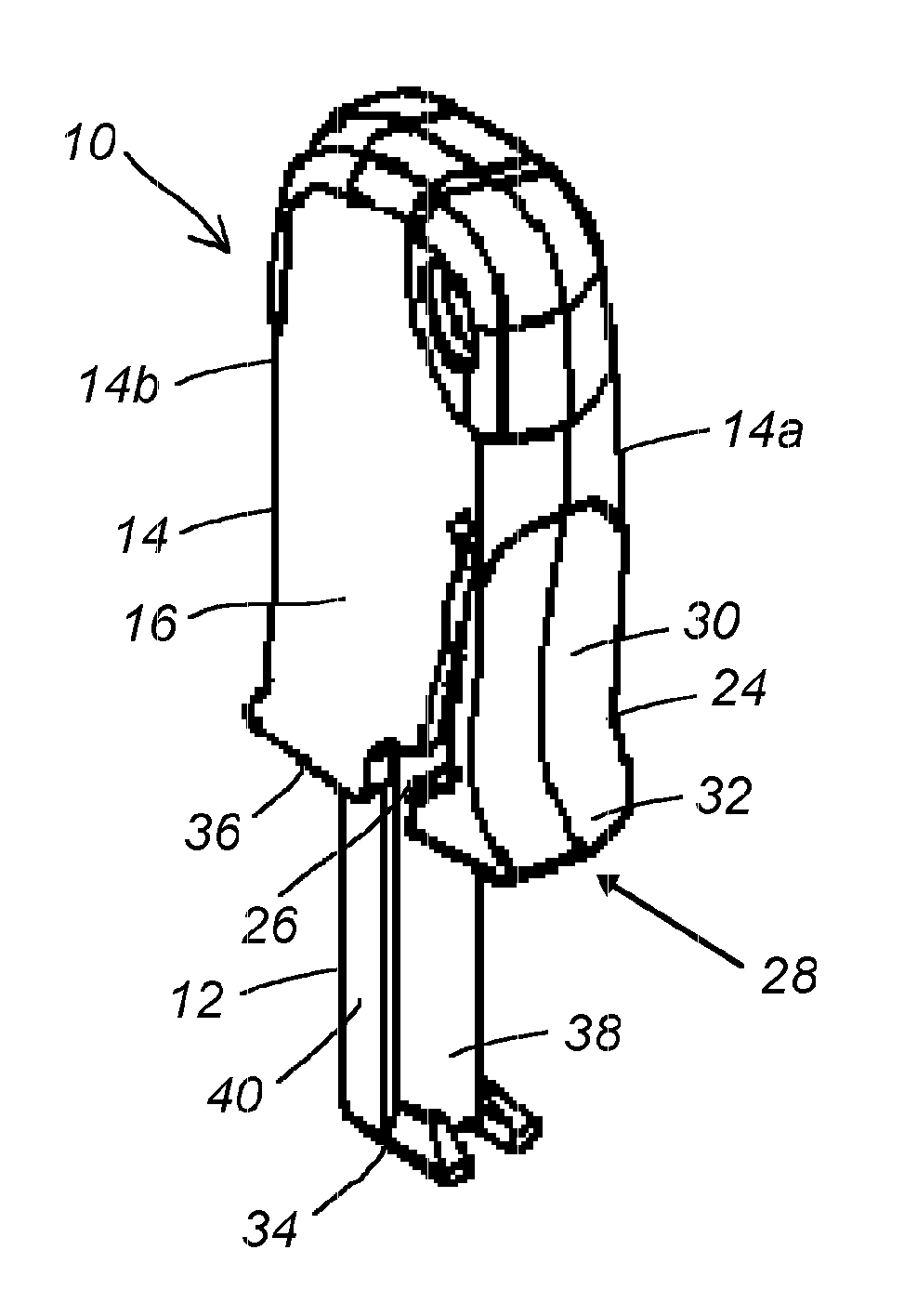
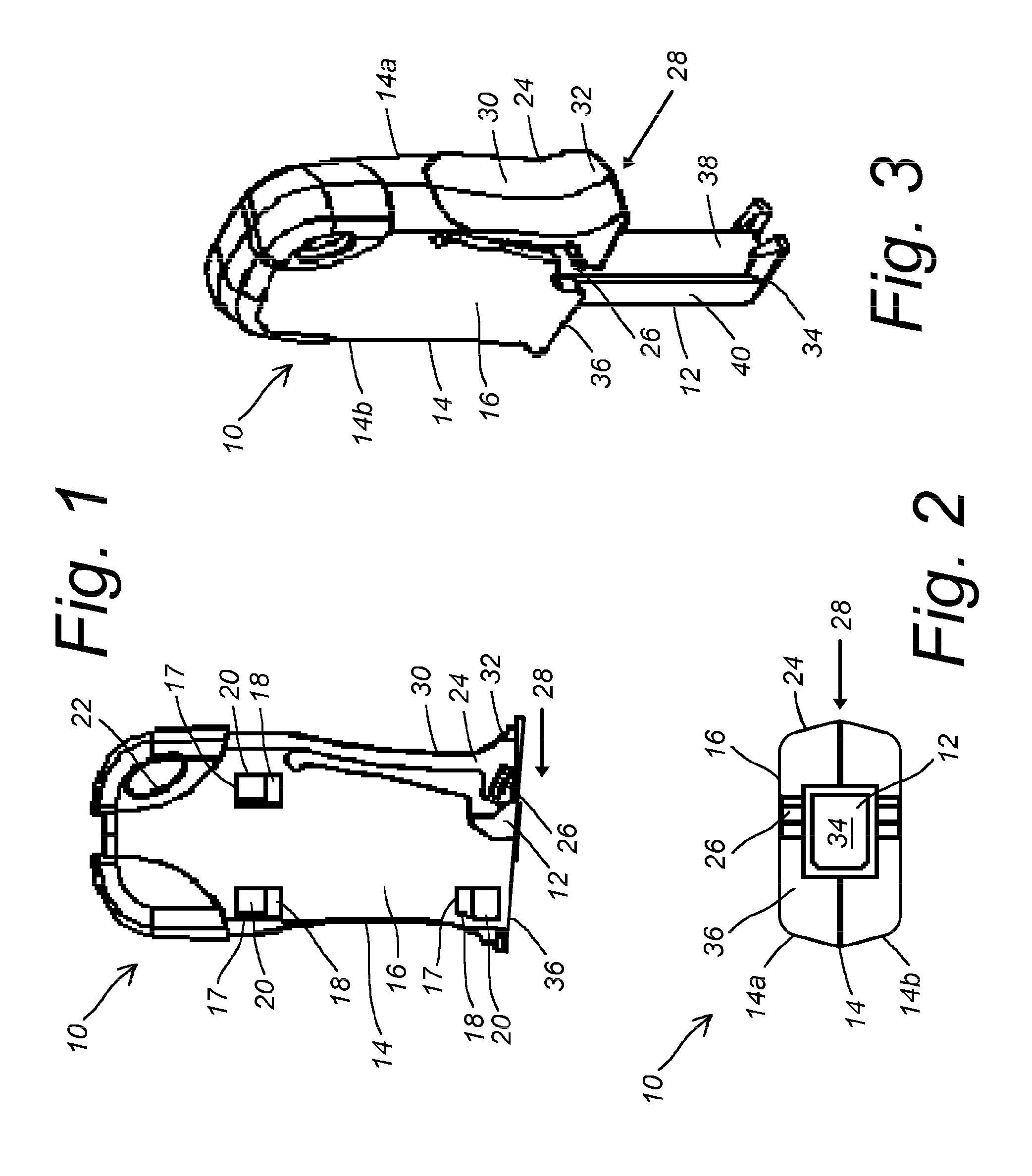
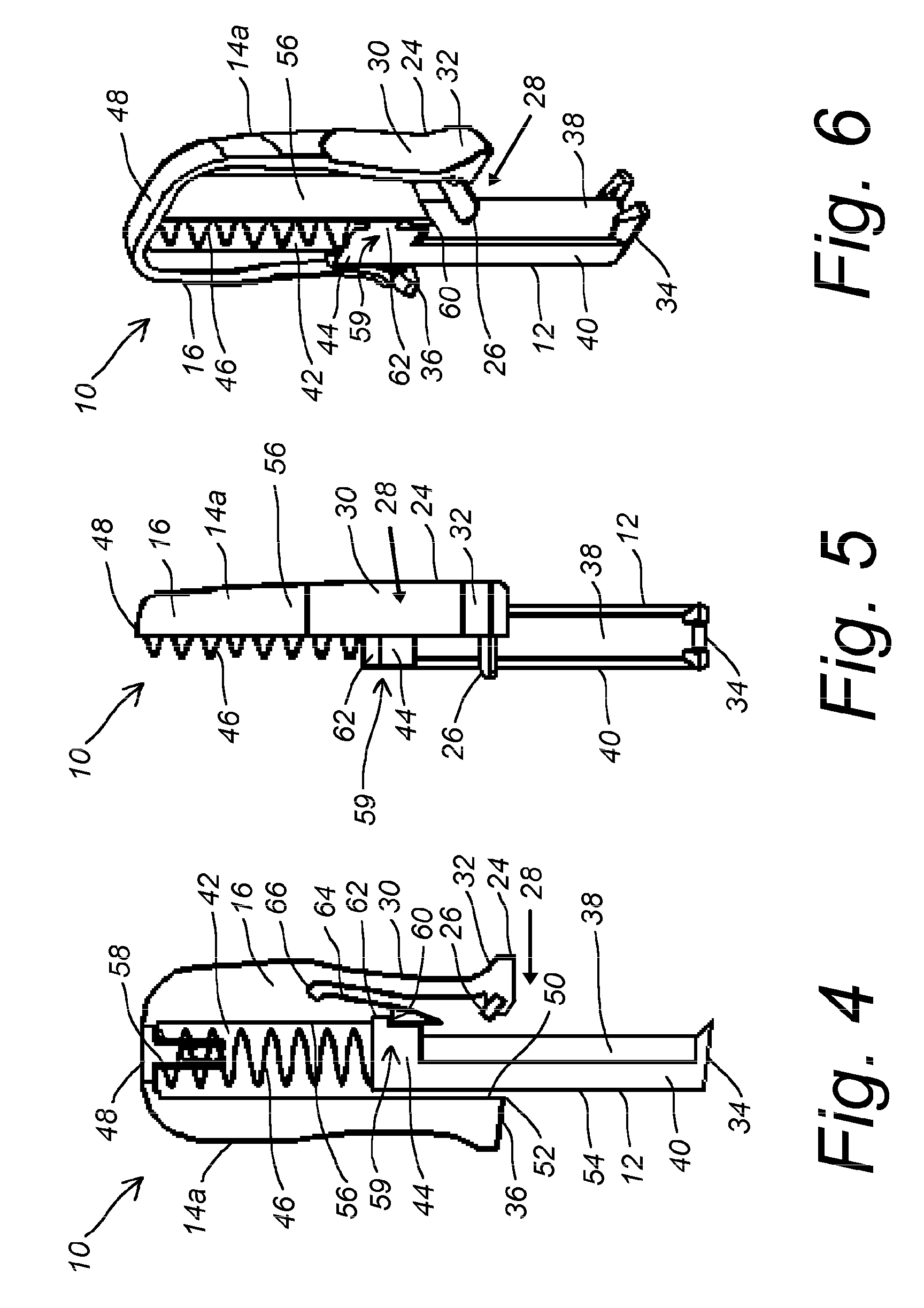
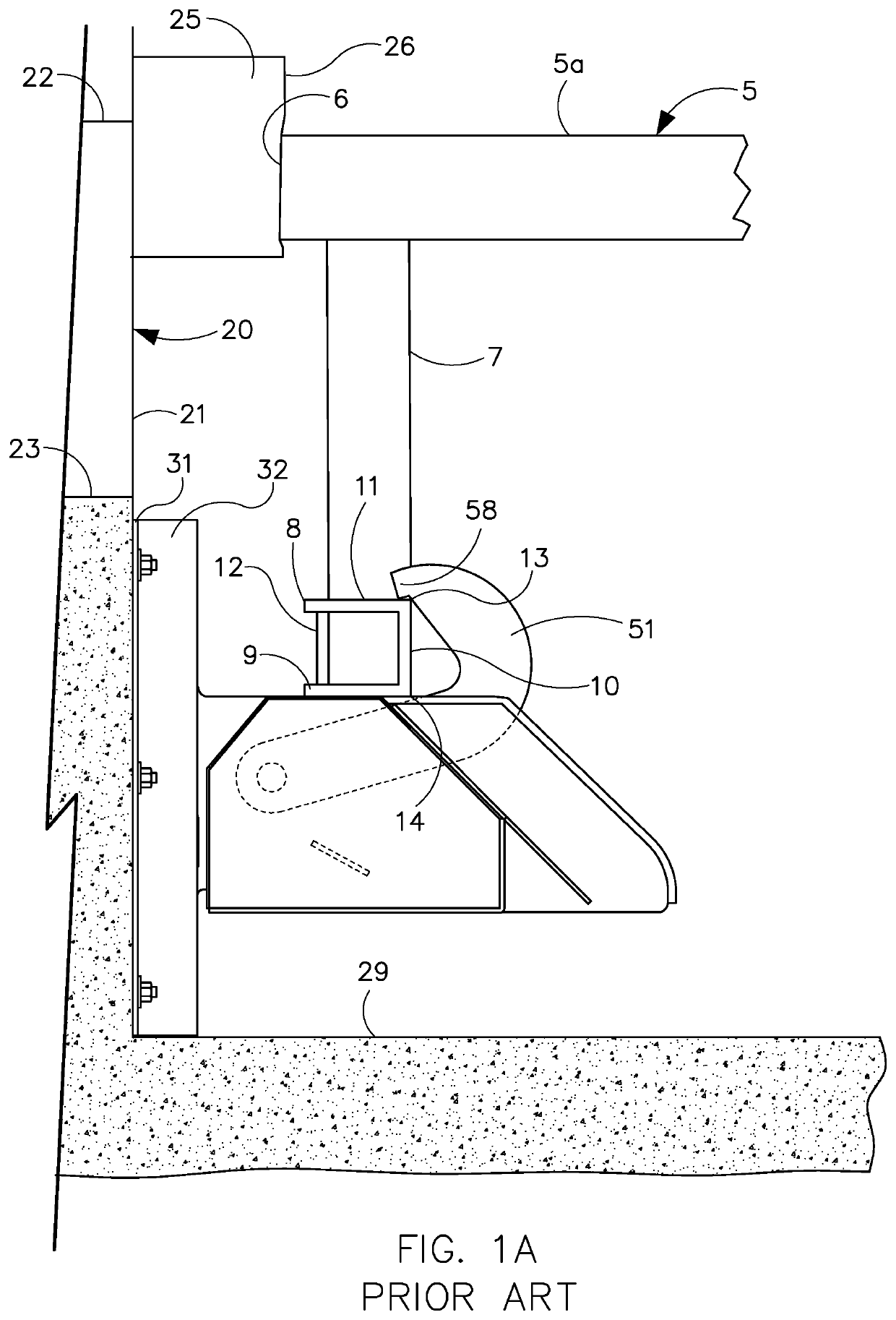
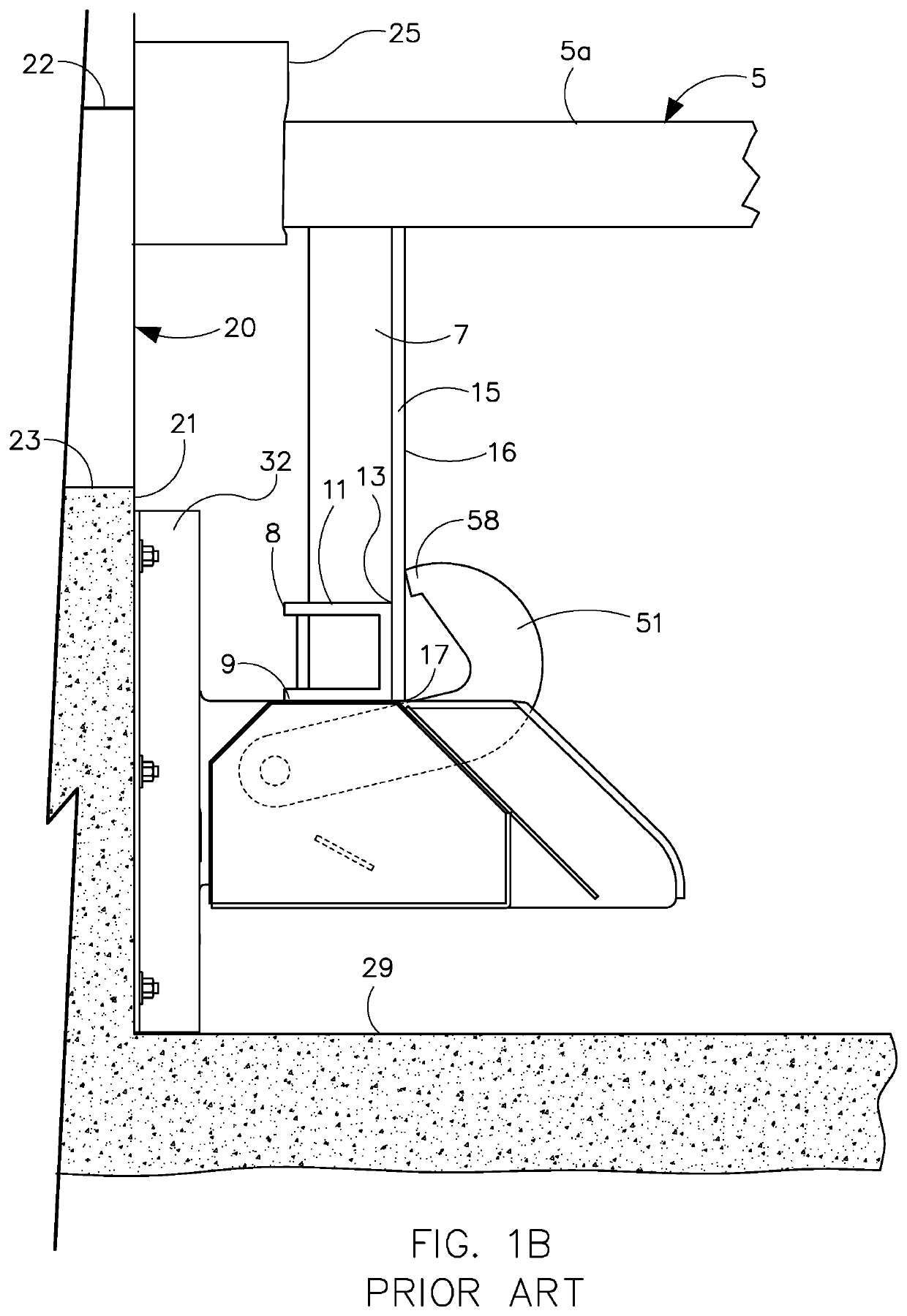
Device and method for treatment of spinal deformity
ActiveUS20080033436A1Shorten the lengthDevice degradationInternal osteosythesisJoint implantsSpinal columnMedicine
The present invention generally relates to methods and devices for treatment of spinal deformity, and in particular to the utilization of at least one implant to either maintain the position of at least one vertebra of a patient to prevent increase in abnormal spinal curvature, to slow progression of abnormal curvature, or to impose at least one corrective displacement and / or rotation on at least one vertebra of a patient so as to incrementally correct abnormal spinal curvature.
Owner:GLOBUS MEDICAL INC
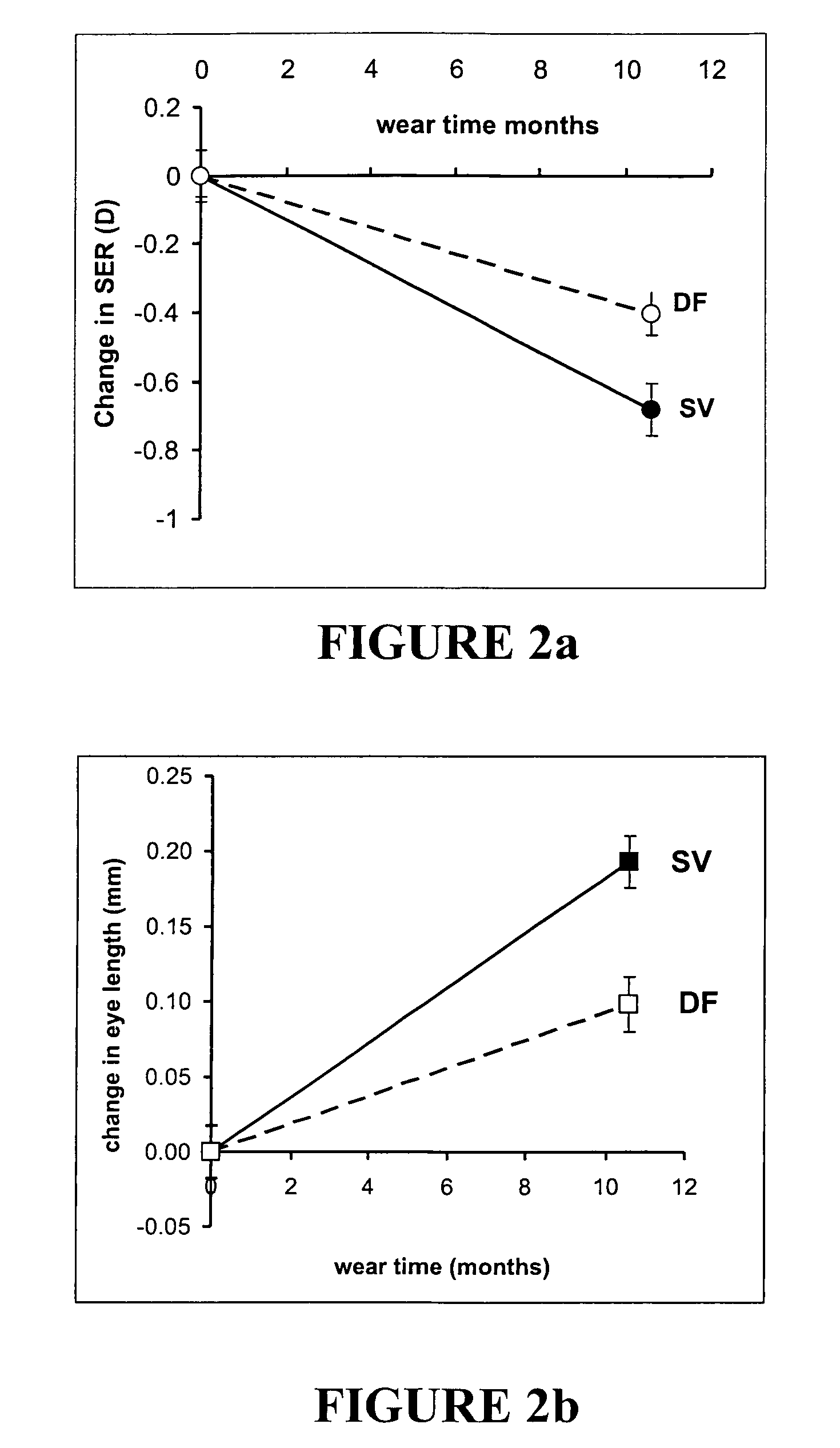
Contact lens and method
ActiveUS20080218687A1Delay myopia progressionStop myopia progressionSpectales/gogglesOptical partsContact lens tintVisual acuity
Owner:AUCKLAND UNISERVICES LTD
Contact lens and method
ActiveUS7832859B2Delay myopia progressionShorten the progressSpectales/gogglesOptical partsContact lens tintVisual acuity
Owner:AUCKLAND UNISERVICES LTD
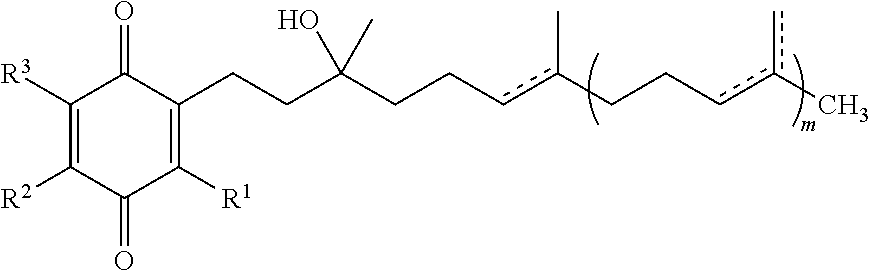
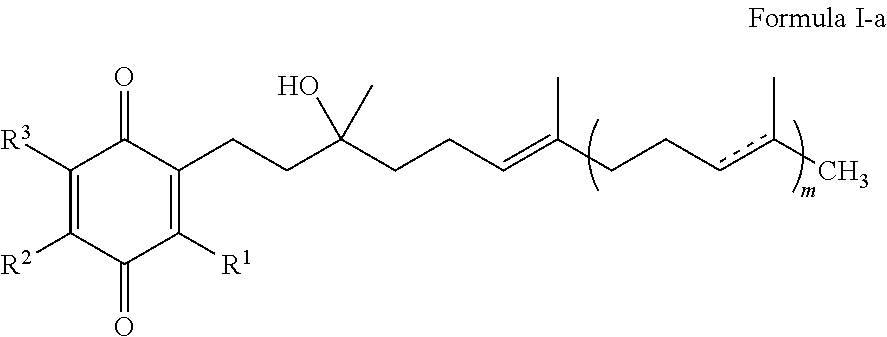
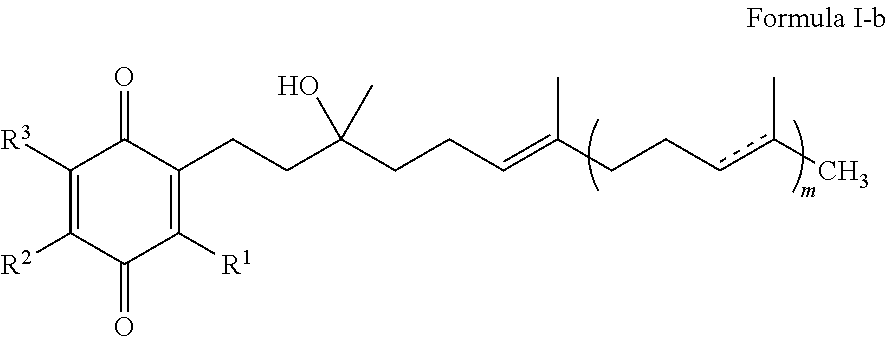
Formulations of quinones for the treatment of ophthalmic diseases
InactiveUS20130109759A1Increase aqueous solubilityAdditional comfort and non irritabilityBiocideSenses disorderQuinoneDisease
A formulation comprising an ophthalmically effective amount of one or more quinones of Formula I. Use of a formulation comprising one or more quinones of Formula I for the prevention, reduction, amelioration or treatment of ophthalmic disorders that are associated with a neurodegenerative or trauma disorder is also discussed. A method of treating or controlling the ocular symptoms associated with neurodegenerative diseases or trauma with a formulation comprising one or more quinones of Formula I is also discussed. A method of treating or controlling the ocular symptoms associated with mitochondrial myopathies with a formulation comprising one or more quinones of Formula I is also discussed.
Owner:EDISON PHARMA
Wound treatment apparatus employing reduced pressure
InactiveUS20080039761A1High cure rateStop progressFinger bandagesNon-adhesive dressingsMedicineBiomedical engineering
Owner:KCI LICENSING INC
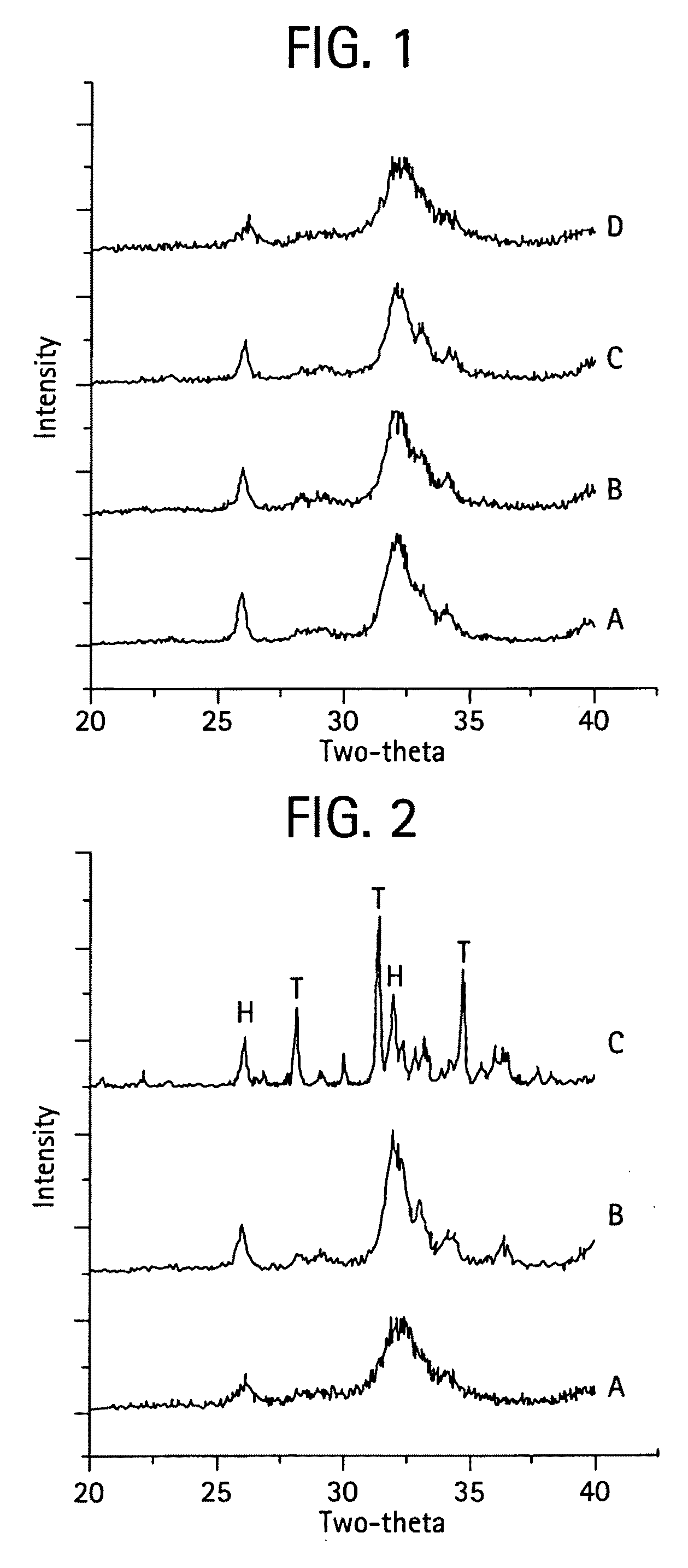
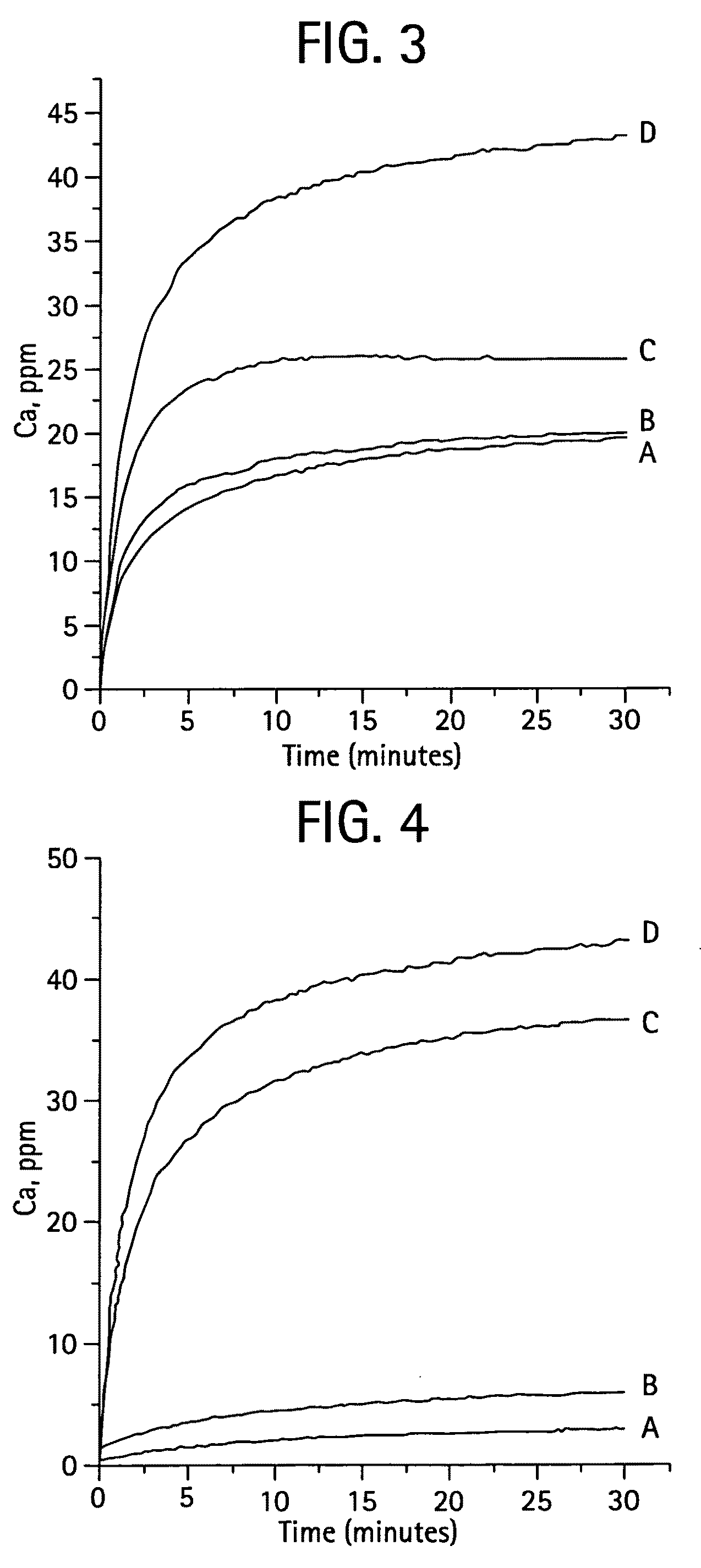
Calcium phosphate-based materials containing zinc, magnesium, fluoride and carbonate
ActiveUS20090068285A1Promotes bone formationMinimize and prevent bone resorptionBiocideInorganic phosphorous active ingredientsDiseaseAntioxidant
The present invention provides novel biomaterials comprising one or more of Mg, Zn and F ions in a carbonate-containing biphasic calcium phosphate (BCP) system. The biomaterial may contain Mg, Zn, F, Mg and Zn, Mg and F, Zn and F, or Mg, Zn and F. The biomaterial may be substantially similar in composition to bone mineral (a carbonate apatite). The biomaterial may feature slow release of Mg, Zn, F, Ca, and P ions. The biphasic calcium phosphate, BCP, may be a mixture of unsubstituted hydroxyapatite (HA) and unsubstituted .-TCP, Ca3(PO4)2. BCP of varying HA / .-TCP ratios may be produced by sintering calcium-deficient apatite, for instance having a Ca / P<1.5, 1.6, 1.67, 1.75 or 1.8 that has been prepared either by a precipitation or by a hydrolysis method or by a solid-state reaction. The amount of each component (by weight %) present in the biomaterials may be as follows: Mg 0.5 to 12 wt %, Zn 1 to 12 wt %, F 0.1 to 4 wt %, calcium 20 to 40 wt %, phosphate 10 to 20 wt %, and carbonate (CO3) 1 to 20 wt %. The biomaterial may further comprise one or more other ion such as strontium, manganese, copper, boron or silicate, or one or more other organic moiety such as a protein, a peptide, or a nutraceutical which may provide antioxidant, anti-bacterial or anti-inflammatory properties. The invention also provides methods of inhibiting bone resorption, methods of treating osteoporosis or delaying the onset of osteoporosis, methods of treating a bone fracture, and methods of inhibiting osteoclast activity. Further, the invention provides methods of treating or reversing bone deficiencies such as bone loss, similar to osteoporosis, caused all or in part by a mineral deficient diet, a disease such as cancer or osteopenia, a treatment such as steroid therapy or radiation therapy, or a physical condition such as immobilization.
Owner:NEW YORK UNIV
Wound treatment apparatus employing reduced pressure
InactiveUS20080312613A1High cure rateStop progressFinger bandagesNon-adhesive dressingsMedicineWound surface
Owner:KCI LICENSING INC
Device and method for treatment of spinal deformity
The present invention generally relates to methods and devices for treatment of spinal deformity, and in particular to the utilization of at least one implant to either maintain the position of at least one vertebra of a patient to prevent increase in abnormal spinal curvature, to slow progression of abnormal curvature, or to impose at least one corrective displacement and / or rotation on at least one vertebra of a patient so as to incrementally correct abnormal spinal curvature.
Owner:GLOBUS MEDICAL INC
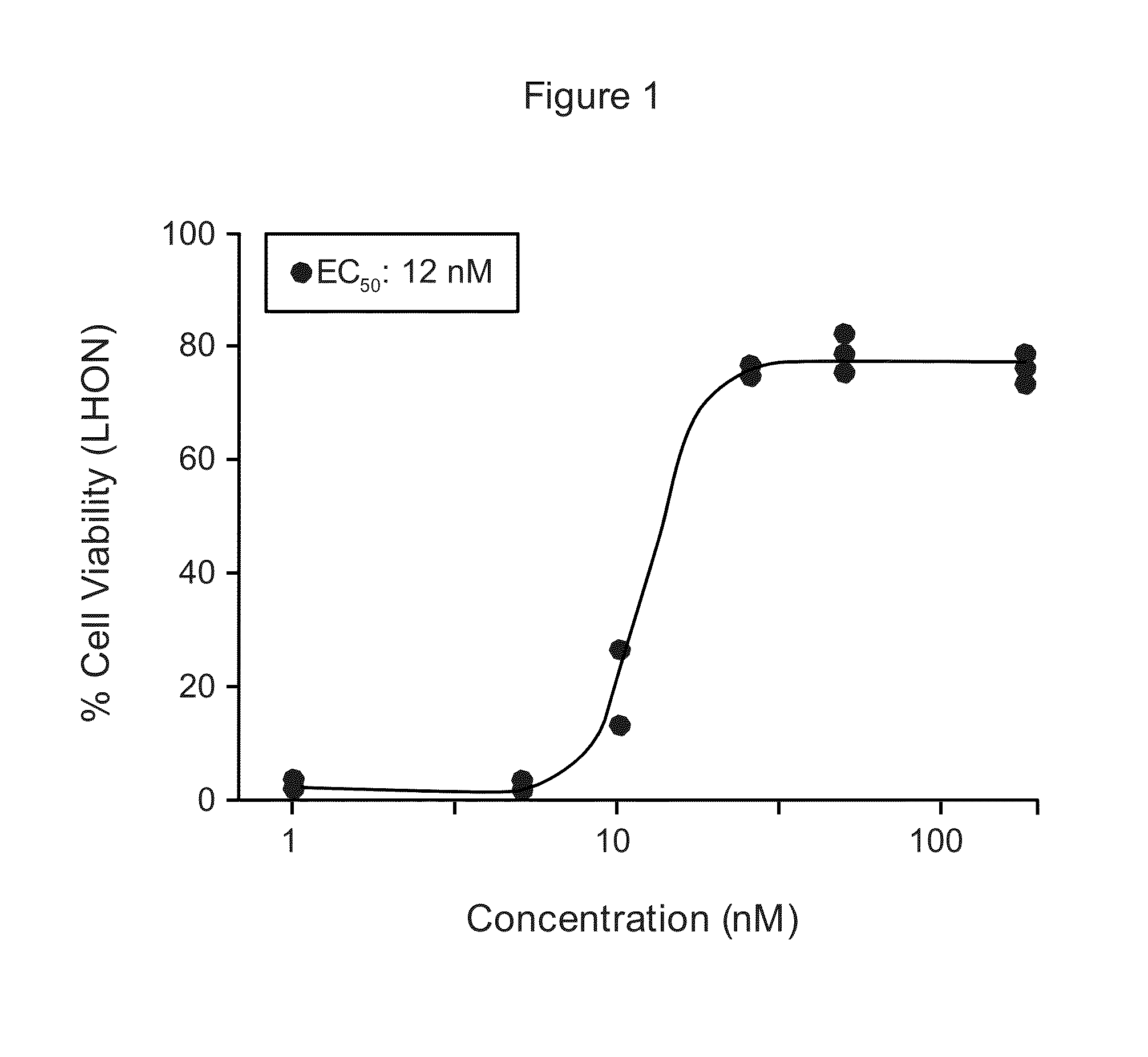
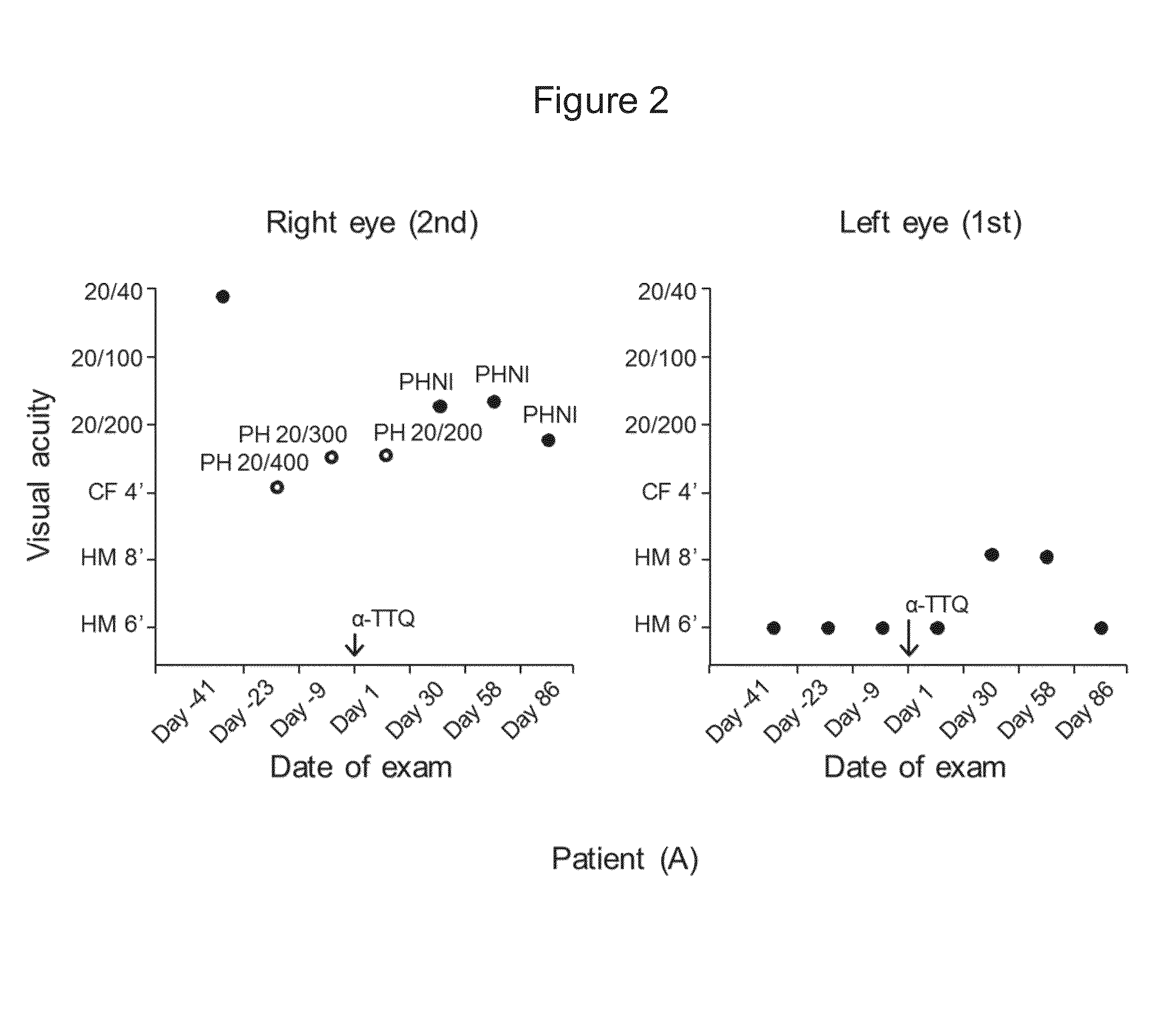
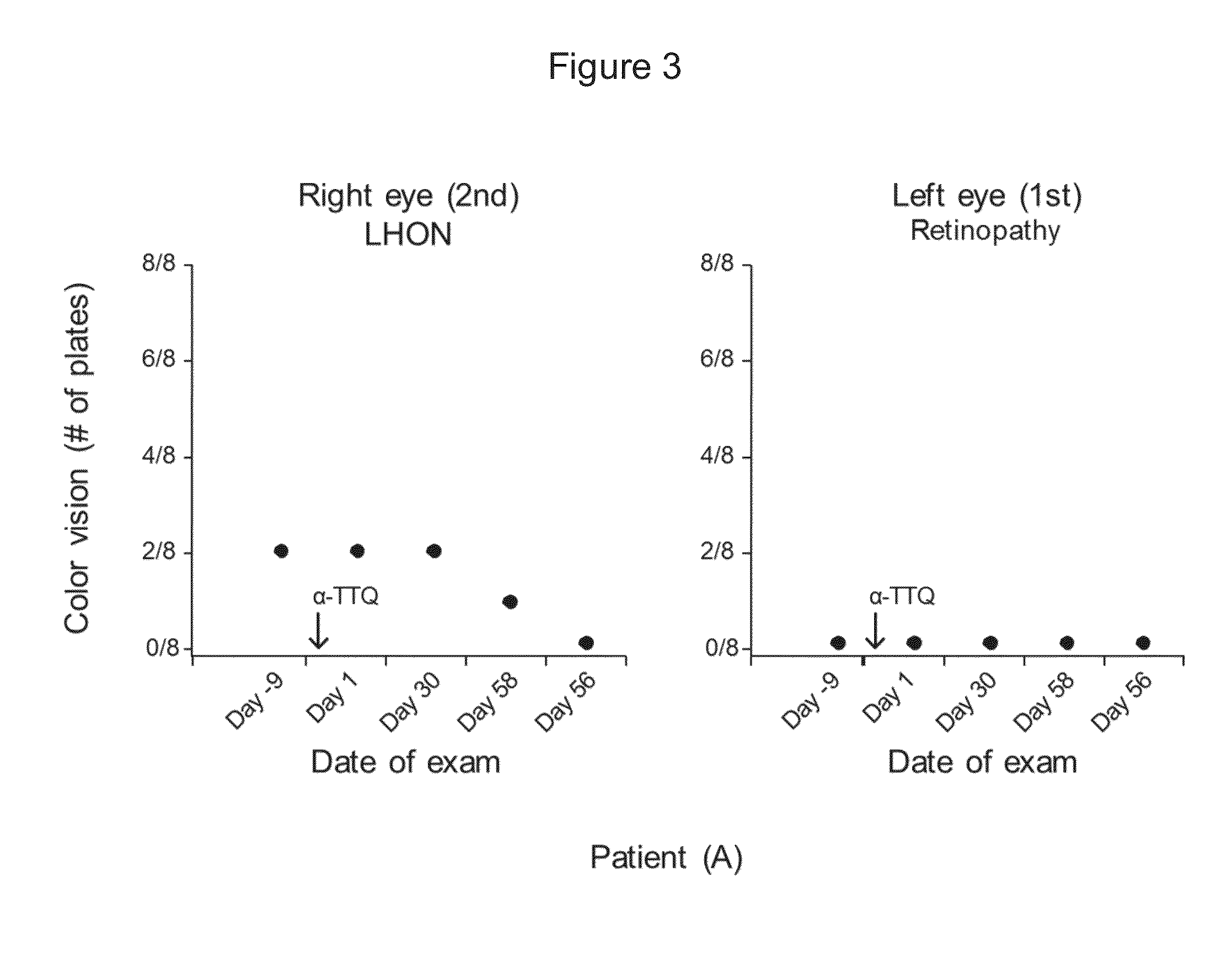
Treatment of leber's hereditary optic neuropathy and dominant optic atrophy with tocotrienol quinones
ActiveUS20140039065A1Stops progression of loss of color visionStop progressBiocideSenses disorderDiseaseAtrophy
The present invention relates to methods of treating Leber's hereditary optic neuropathy and dominant optic atrophy with tocotrienol quinones, including alpha-tocotrienol quinone, in order to alleviate symptoms of the disease.
Owner:PTC THERAPEUTICS INC
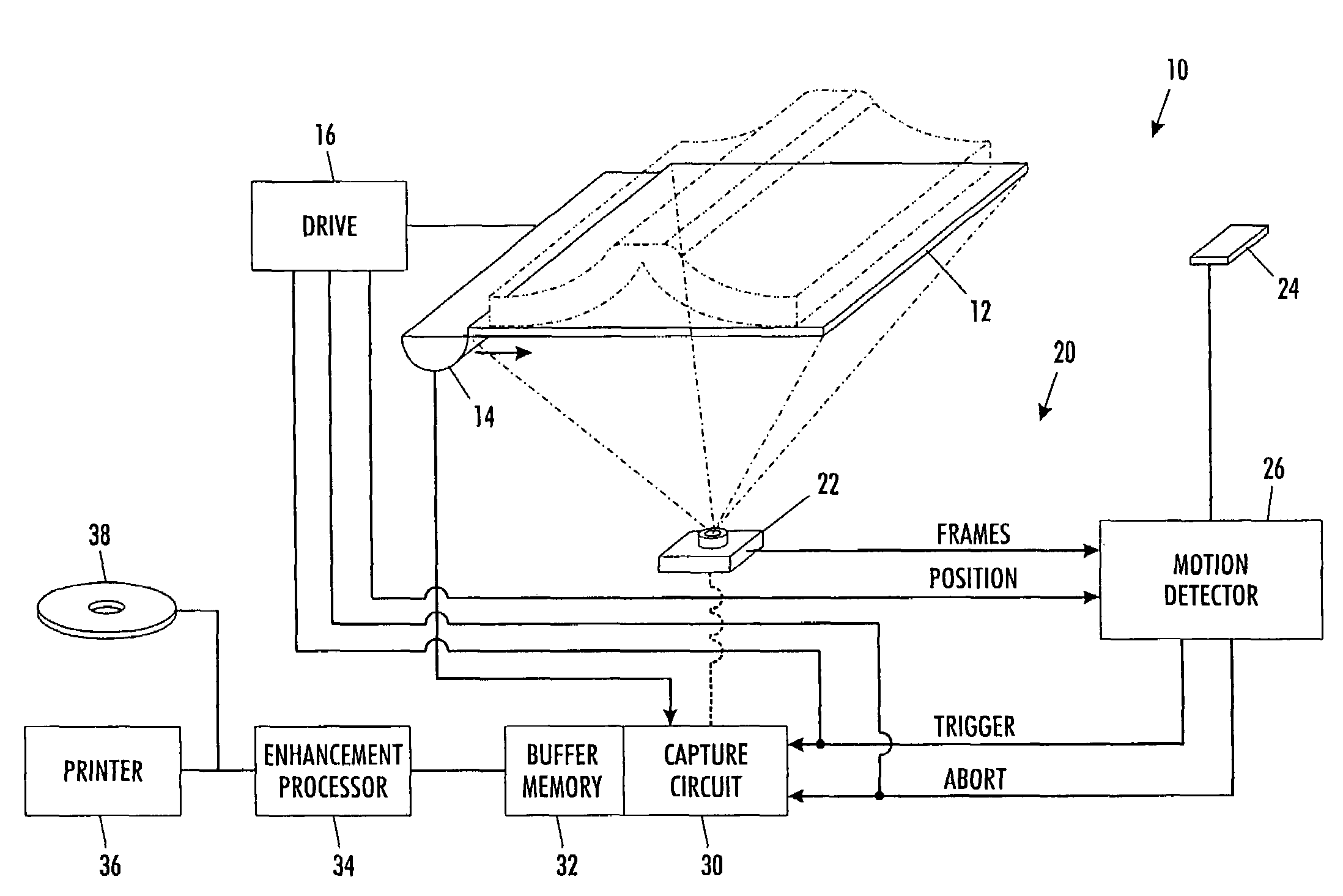
Controlling scanning and copying devices through implicit gestures
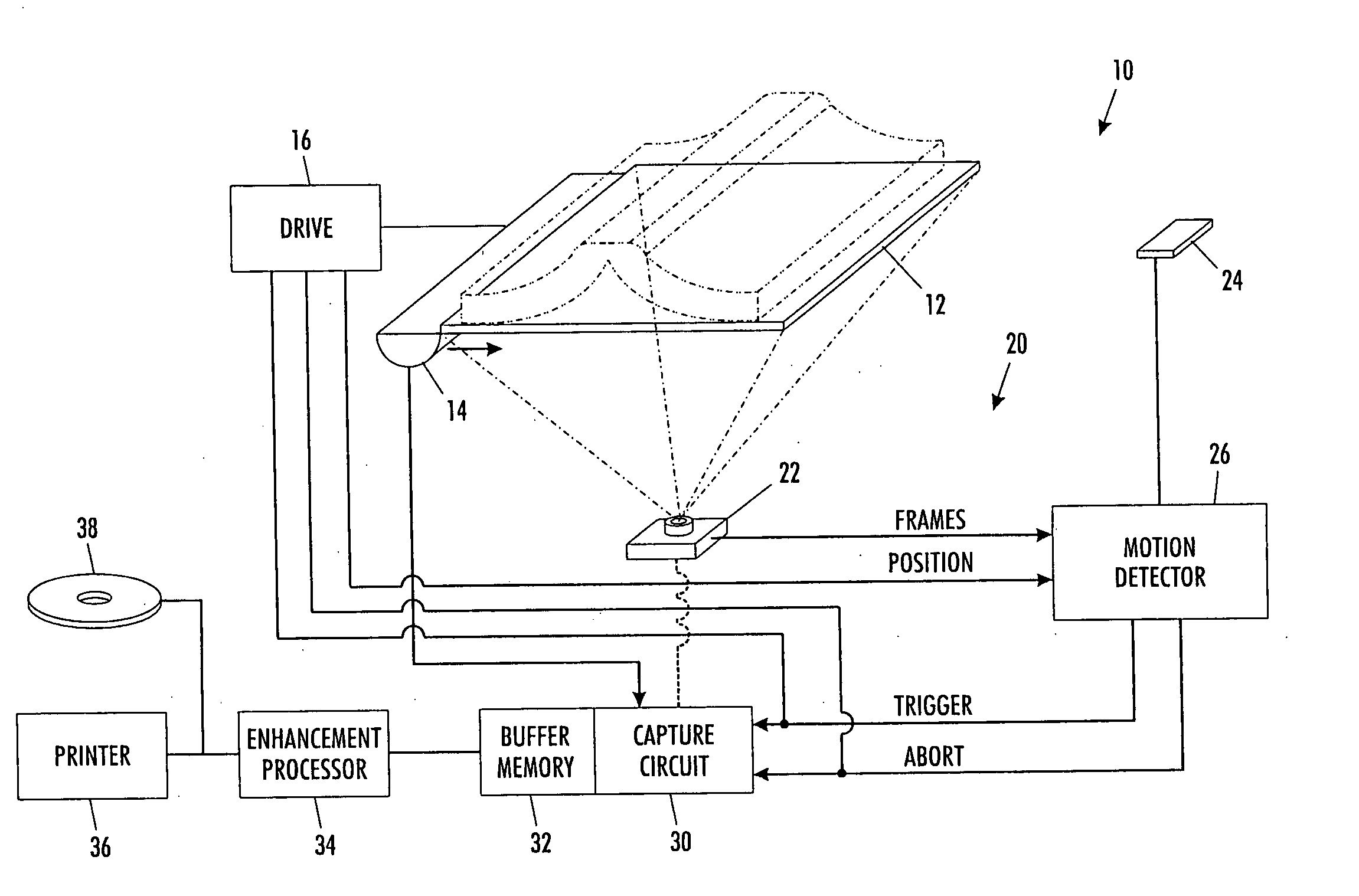
ActiveUS7593144B2Automatically performRelieves operatorCharacter and pattern recognitionElectrographic process apparatusMotion detectorComputer science
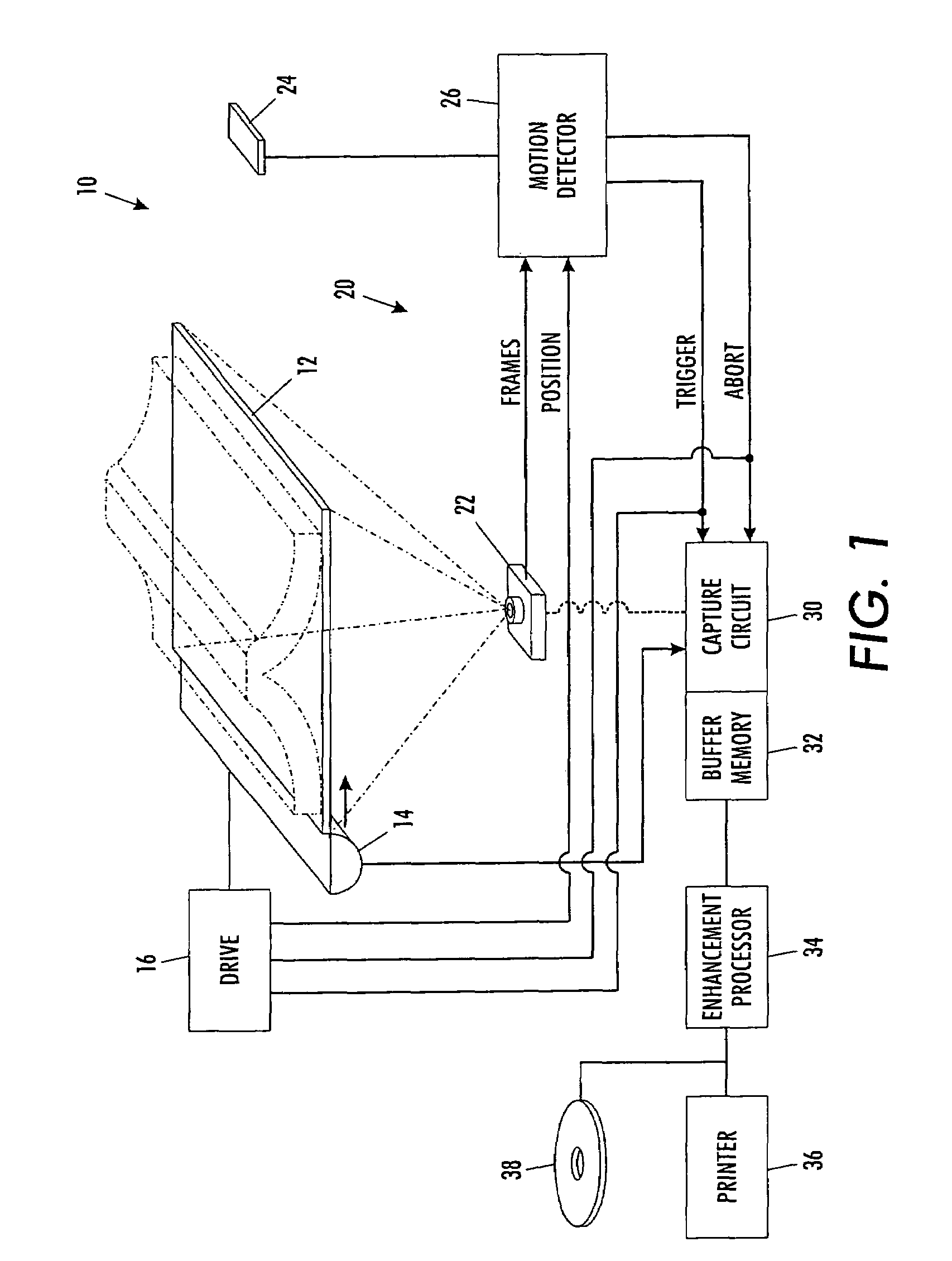
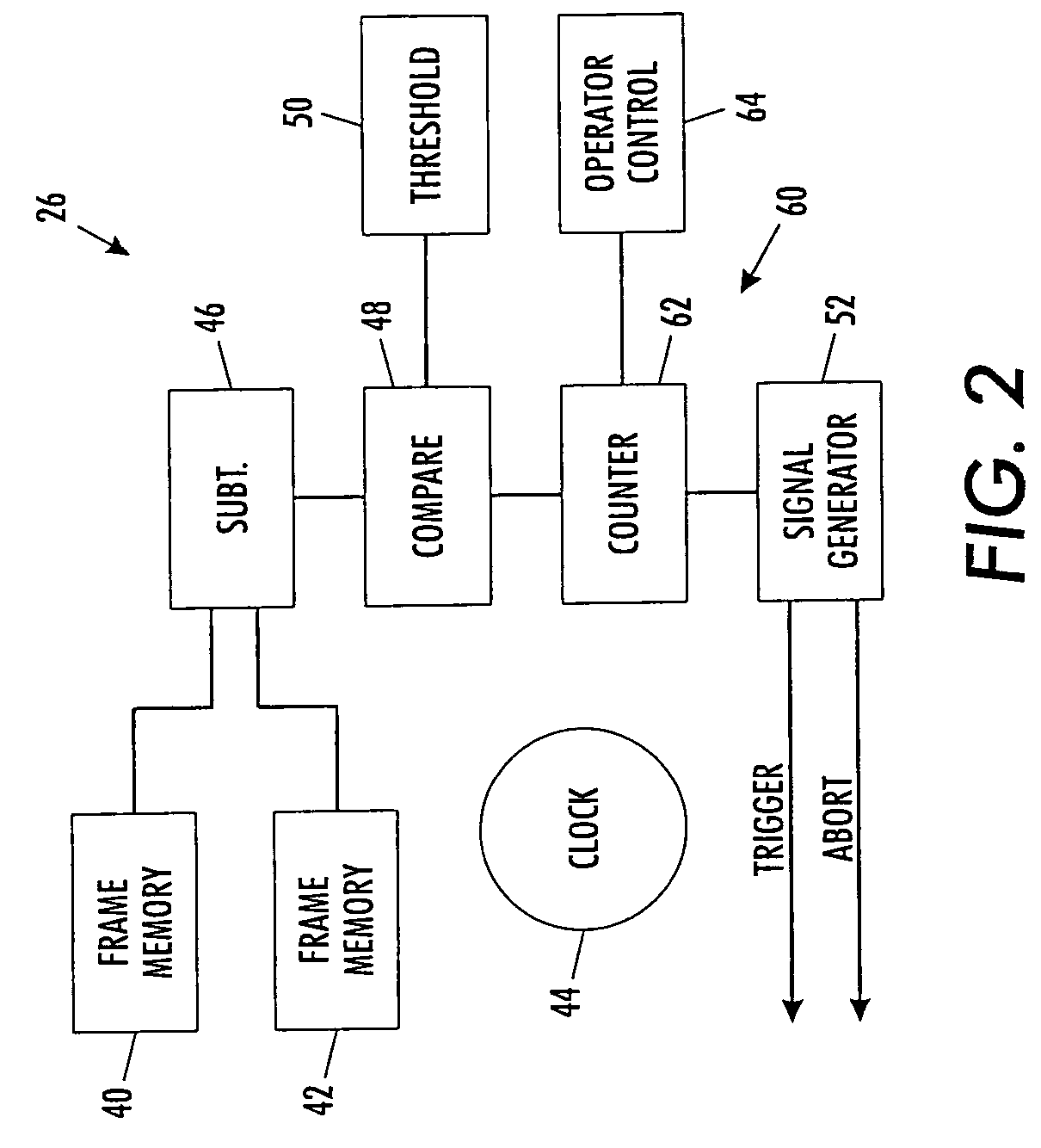
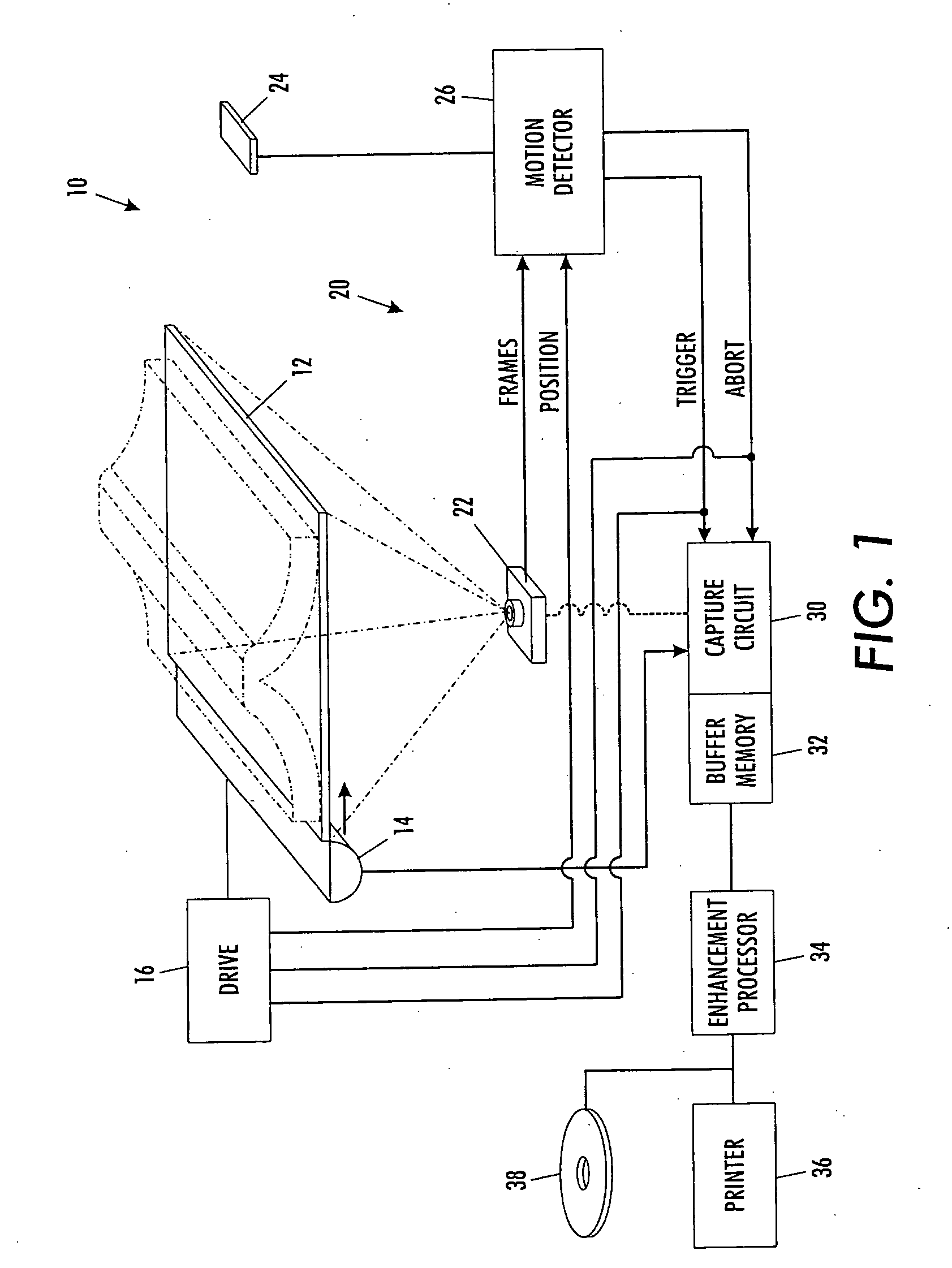
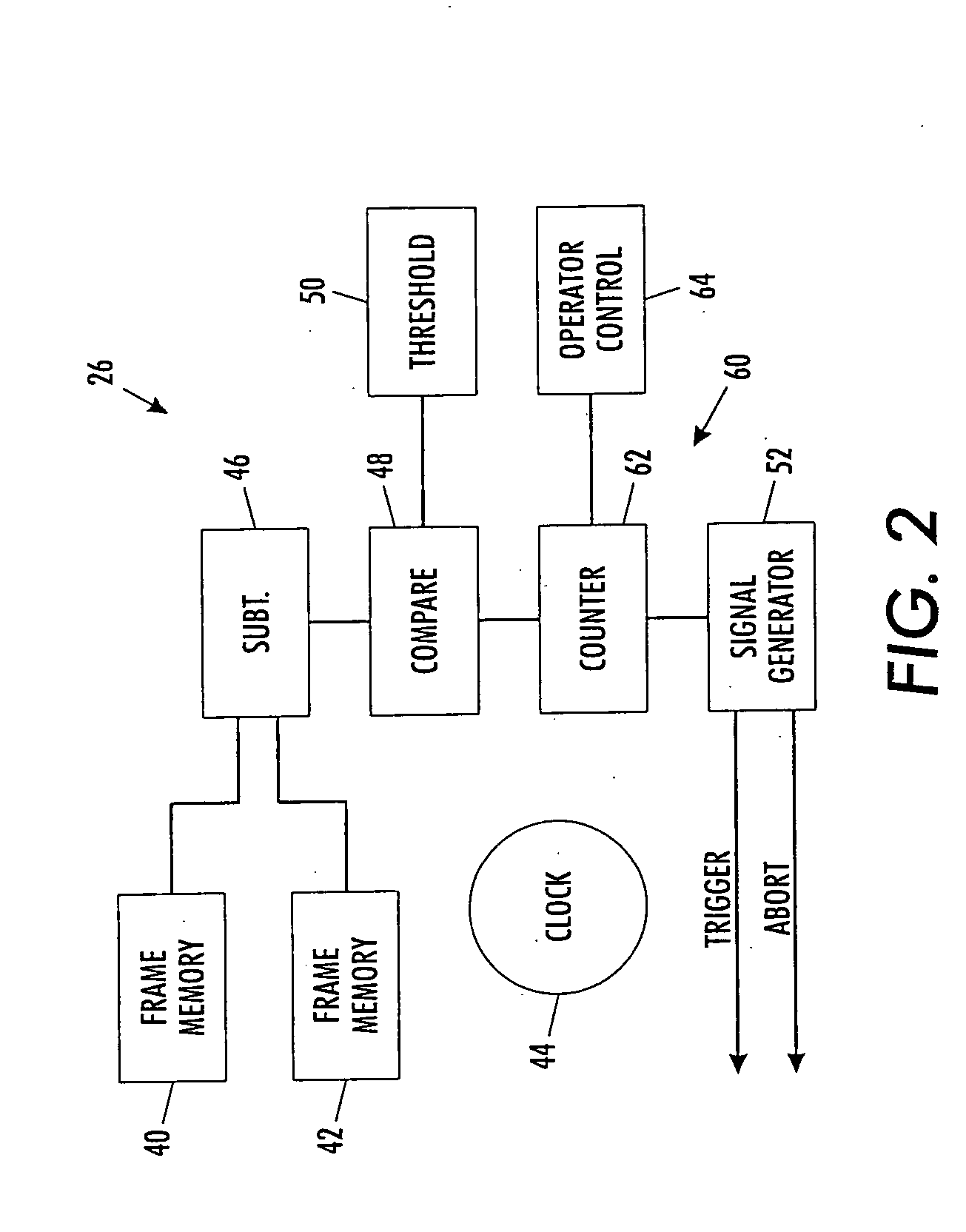
A scanning machine (10) includes a scan surface (12) for receiving a page or pages to be scanned. A motion detector (20) monitors the copy surface to detect movement of the pages. In response to detecting a cessation of motion on the scan surface, the motion detector generates a trigger signal which causes a capture circuit (30) to capture an image of a page on the scan surface. If, during the capture of the page image, the motion detector detects motion of the page, the motion detector generates an abort signal which terminates capture of the image and empties a buffer memory (32). Once cessation of motion of the page is again detected, another trigger signal is issued and another attempt is made to capture an image. In this manner, scanning is started and stopped by natural, simple gestures without the operator pushing buttons.
Owner:XEROX CORP
Apoptosis-inducing agent
InactiveCN103619355AStop progressAnticipating the effect of retreatPeptide/protein ingredientsGenetic material ingredientsDiseaseActive component
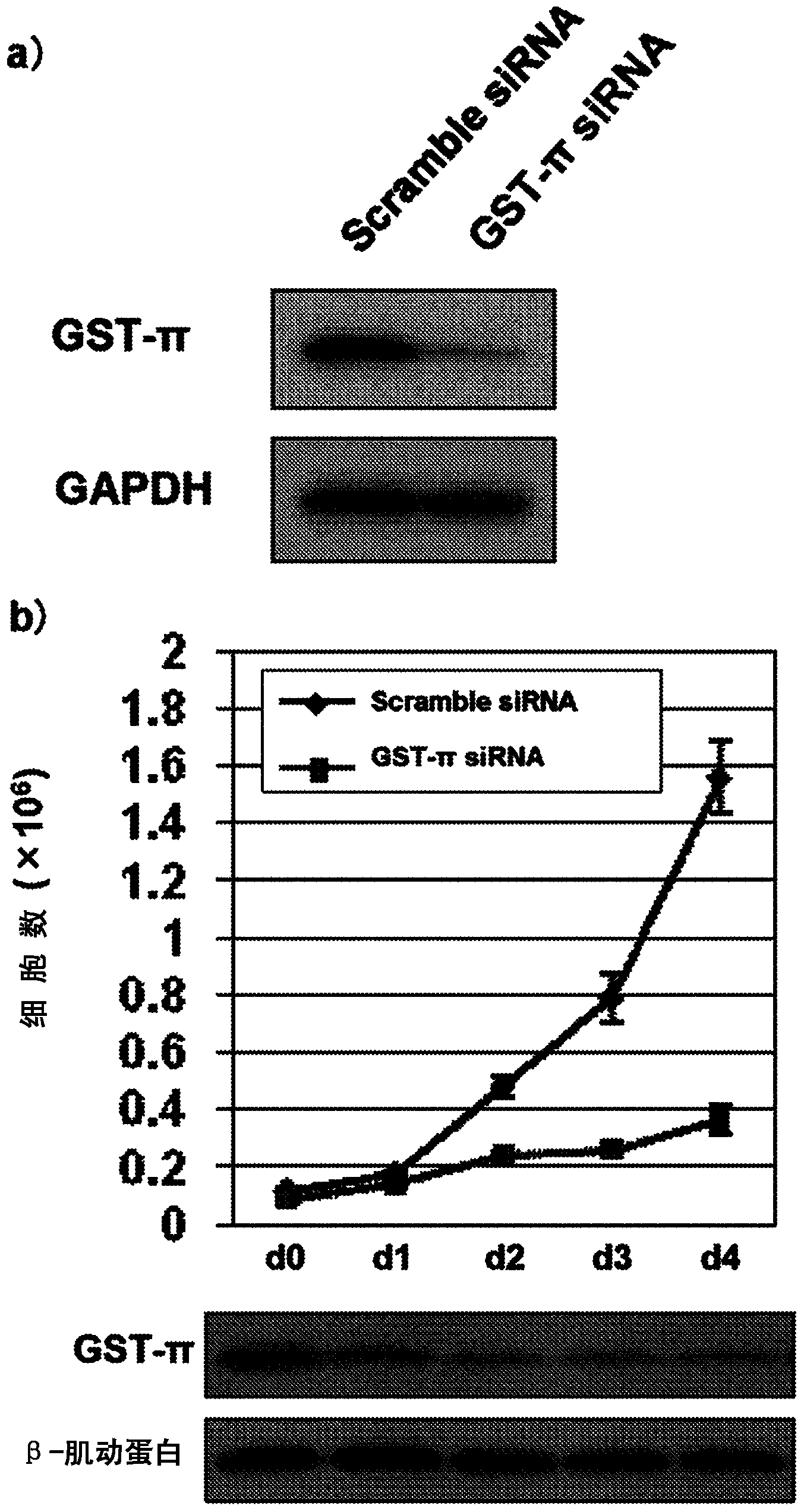
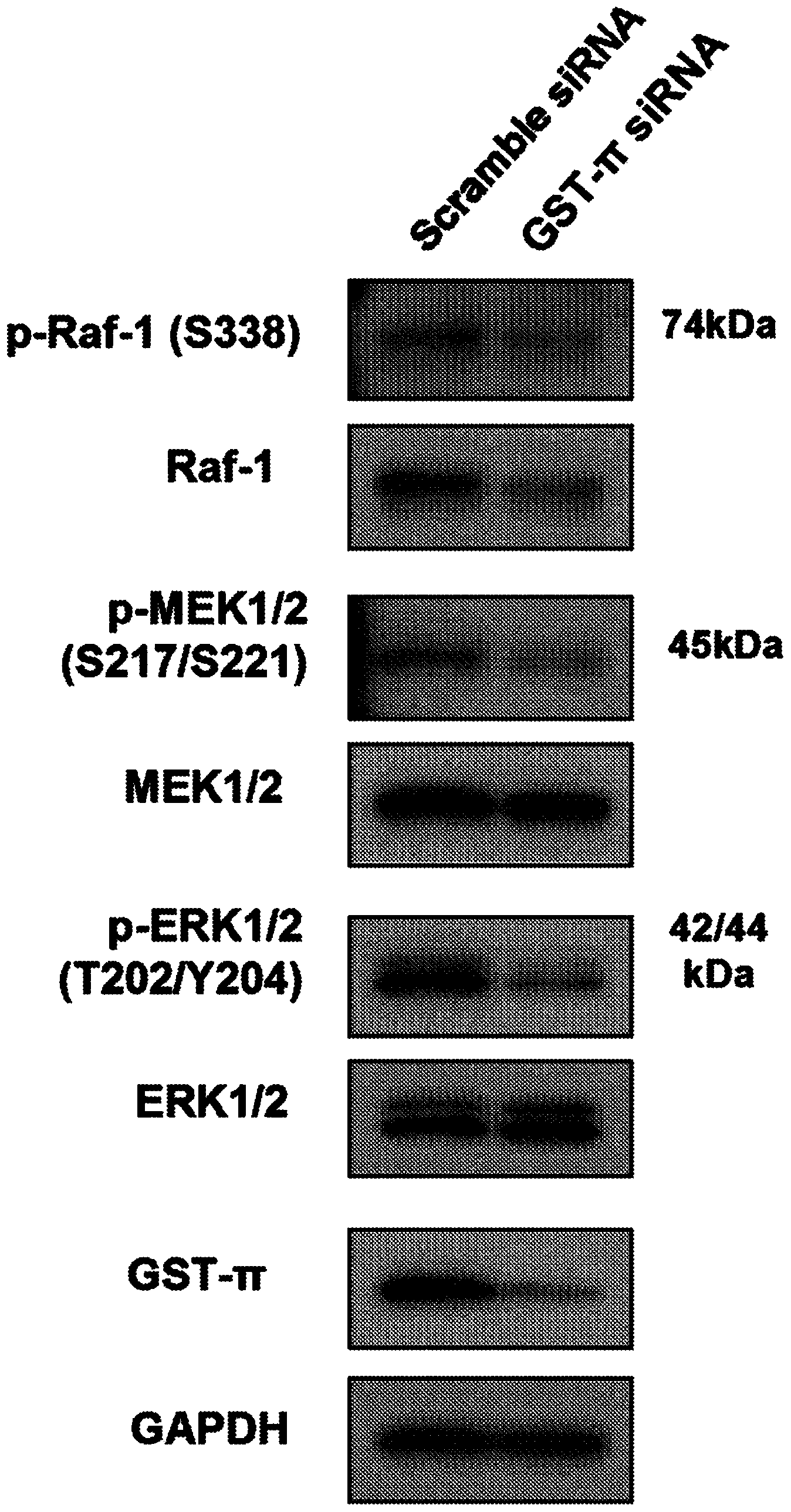
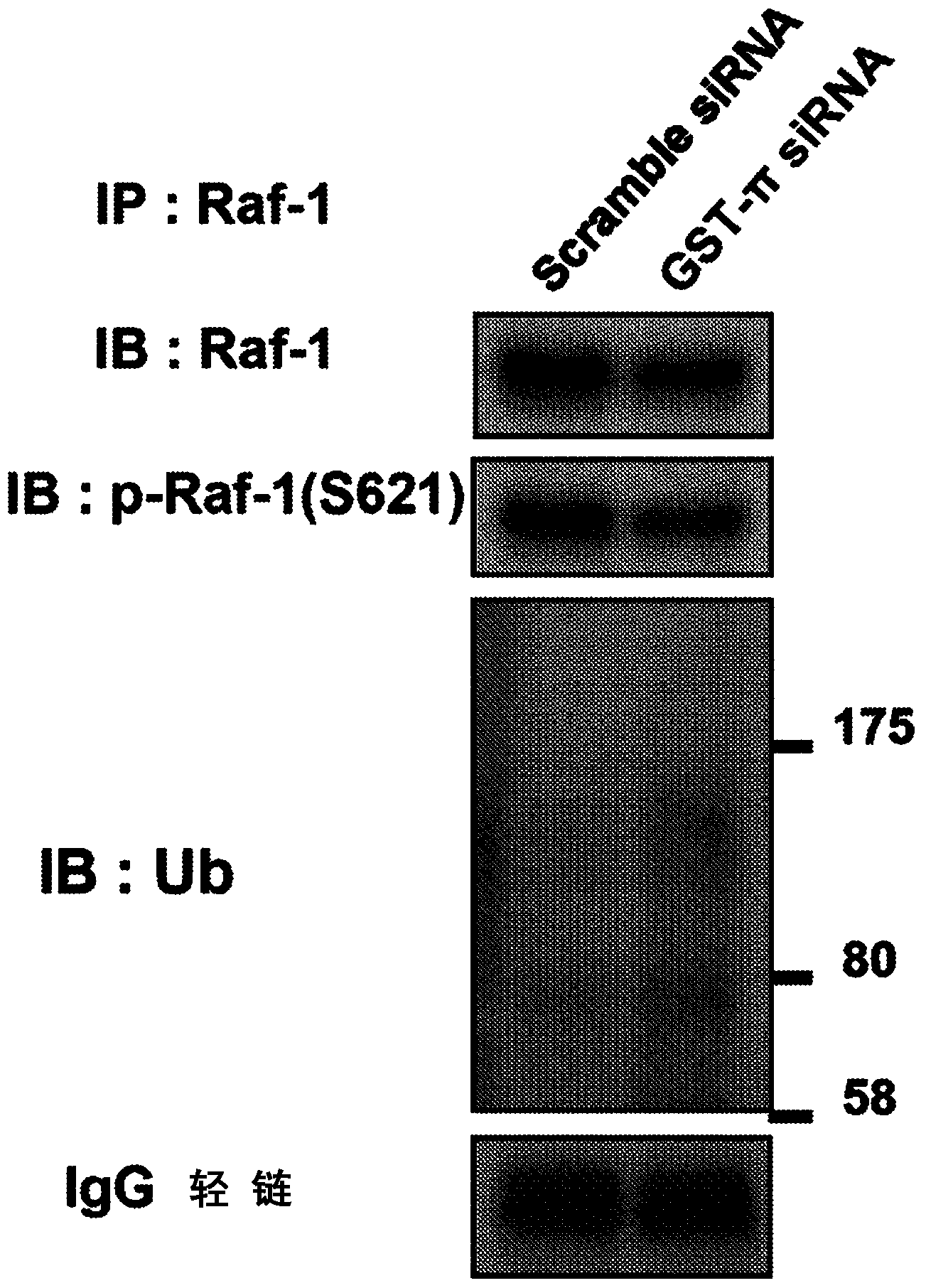
This invention relates to a novel use of GST-p and GST-p suppressing agents; an apoptosis-inducing agent containing as active components a drug that suppresses GST-p and a drug that suppresses autophagy; a medical composition containing said agent; and a method using said medical composition for treating diseases associated with abnormal apoptosis.
Owner:NITTO DENKO CORP
Apparatus and System for Testing Memory
InactiveUS20080057483A1Stop progressEasy to useElectrical appliancesTeaching apparatusTest facilityMobile phone
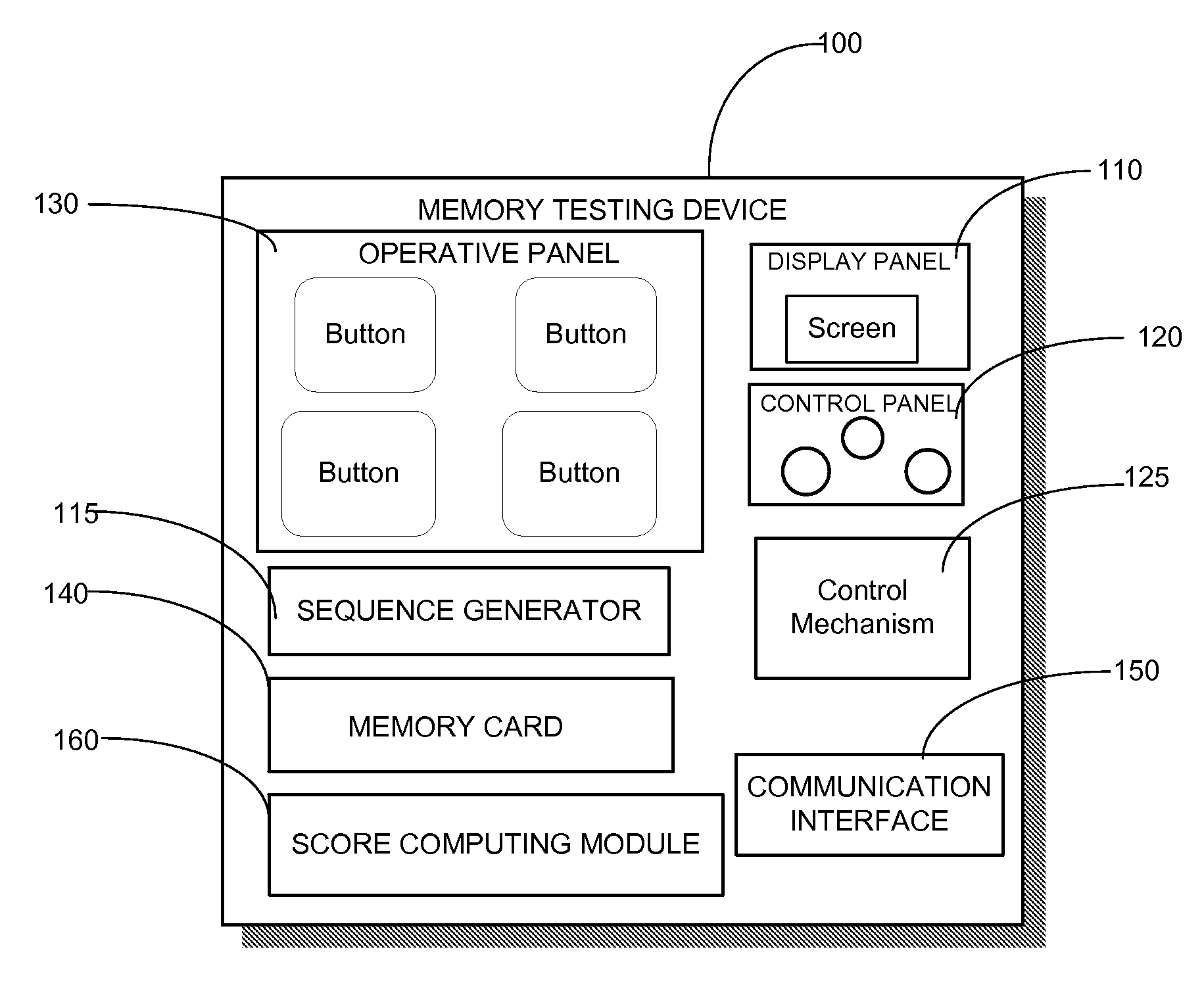
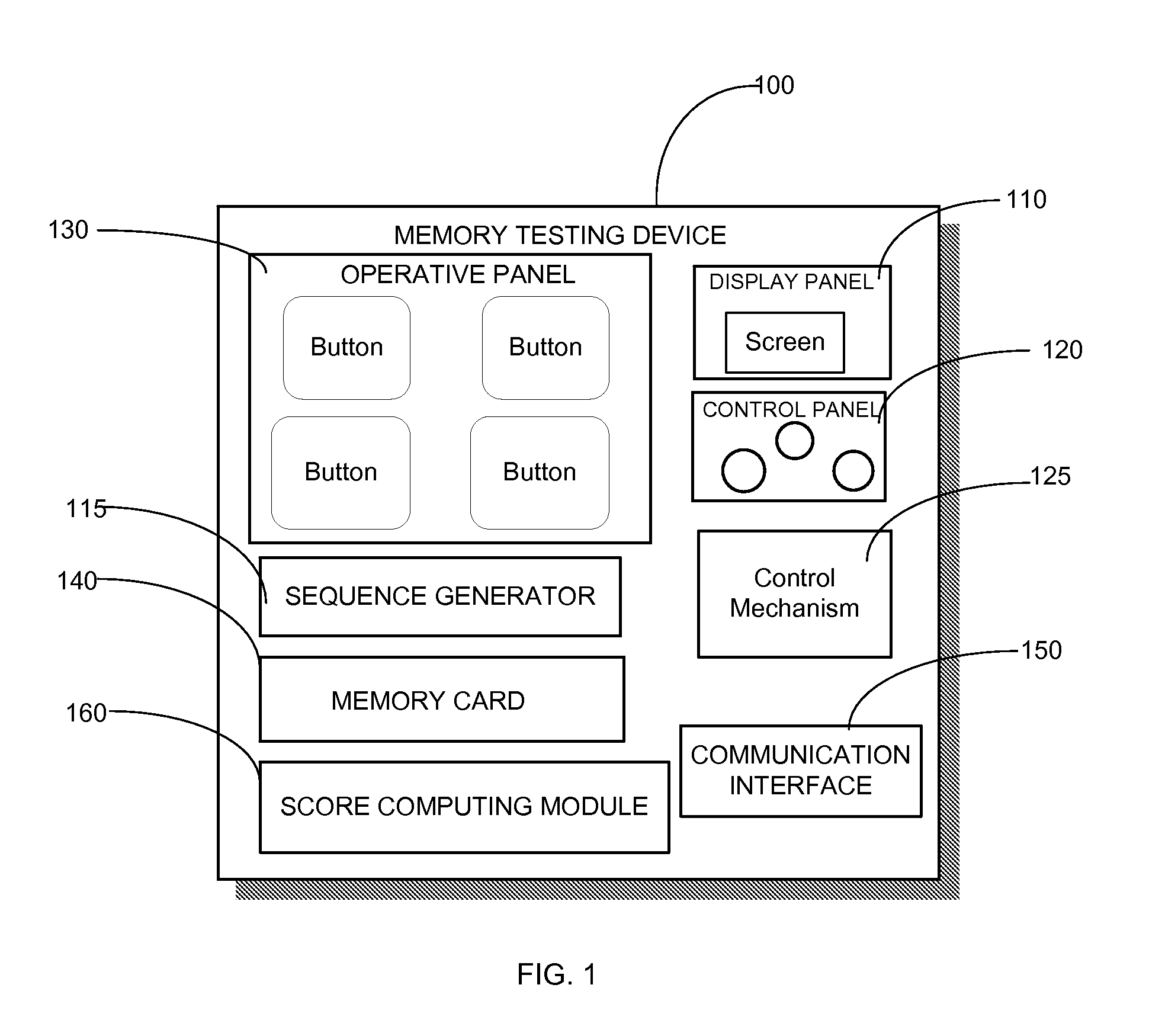
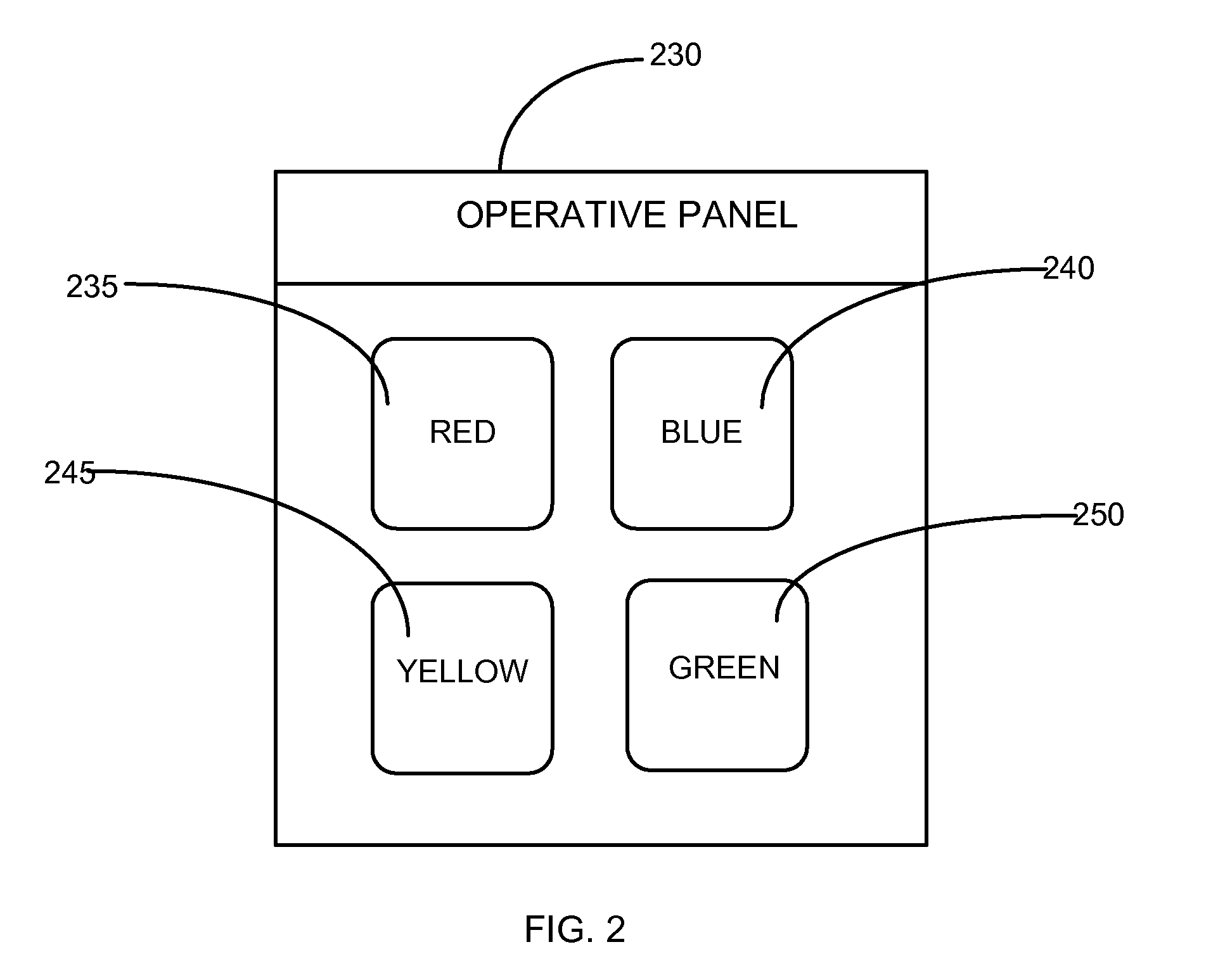
Different memory testing devices, systems, and methods for conducting memory tests, and for storing and displaying test data and trends are disclosed. The tests generally are based on generating a random sequence of signals, displaying the sequence to an examinee, the examinee memorizing the sequence, and inputting the sequence into the testing device. The signals could have visual, auditory, positional, and / or tactile components. The signals could also be alphanumeric strings.The testing device measures test outcome variables, computes a composite memory score based on the test outcome variables, records and displays the stored test data. The testing device may also include a communication interface for periodically transferring the stored test data to a database residing on a remote server displaying the test data of the examinee on a website through an internet connectionThe testing device need not be a physical device. Instead, it is possible to implement the functionality of the testing device in a virtual device which is basically a software application, installed and used on a personal computer, a mobile phone, a PDA, etc.
Owner:AVIDAN LAWRENCE H
Calcium phosphate-based materials containing zinc, magnesium, fluoride and carbonate
InactiveUS7419680B2Reduce developmentStop progressBiocideOrganic active ingredientsCalcium biphosphateMedicine
Compositions useful in the prevention and treatment of osteoporosis and for bone and fracture repair. Slow-releasing calcium phosphate-based materials are disclosed, incorporating Mg, Zn, F and carbonate, which will promote bone formation and inhibit bone resorption and thus be agents for the cited uses.
Owner:NEW YORK UNIV
Controlling scanning and copying devices through implicit gestures
ActiveUS20060291004A1Easy to scanElimination of blurred and defective copyCharacter and pattern recognitionElectrographic process apparatusMotion detectorComputer science
A scanning machine (10) includes a scan surface (12) for receiving a page or pages to be scanned. A motion detector (20) monitors the copy surface to detect movement of the pages. In response to detecting a cessation of motion on the scan surface, the motion detector generates a trigger signal which causes a capture circuit (30) to capture an image of a page on the scan surface. If, during the capture of the page image, the motion detector detects motion of the page, the motion detector generates an abort signal which terminates capture of the image and empties a buffer memory (32). Once cessation of motion of the page is again detected, another trigger signal is issued and another attempt is made to capture an image. In this manner, scanning is started and stopped by natural, simple gestures without the operator pushing buttons.
Owner:XEROX CORP
Open-close member control apparatus and method for controlling open-close member
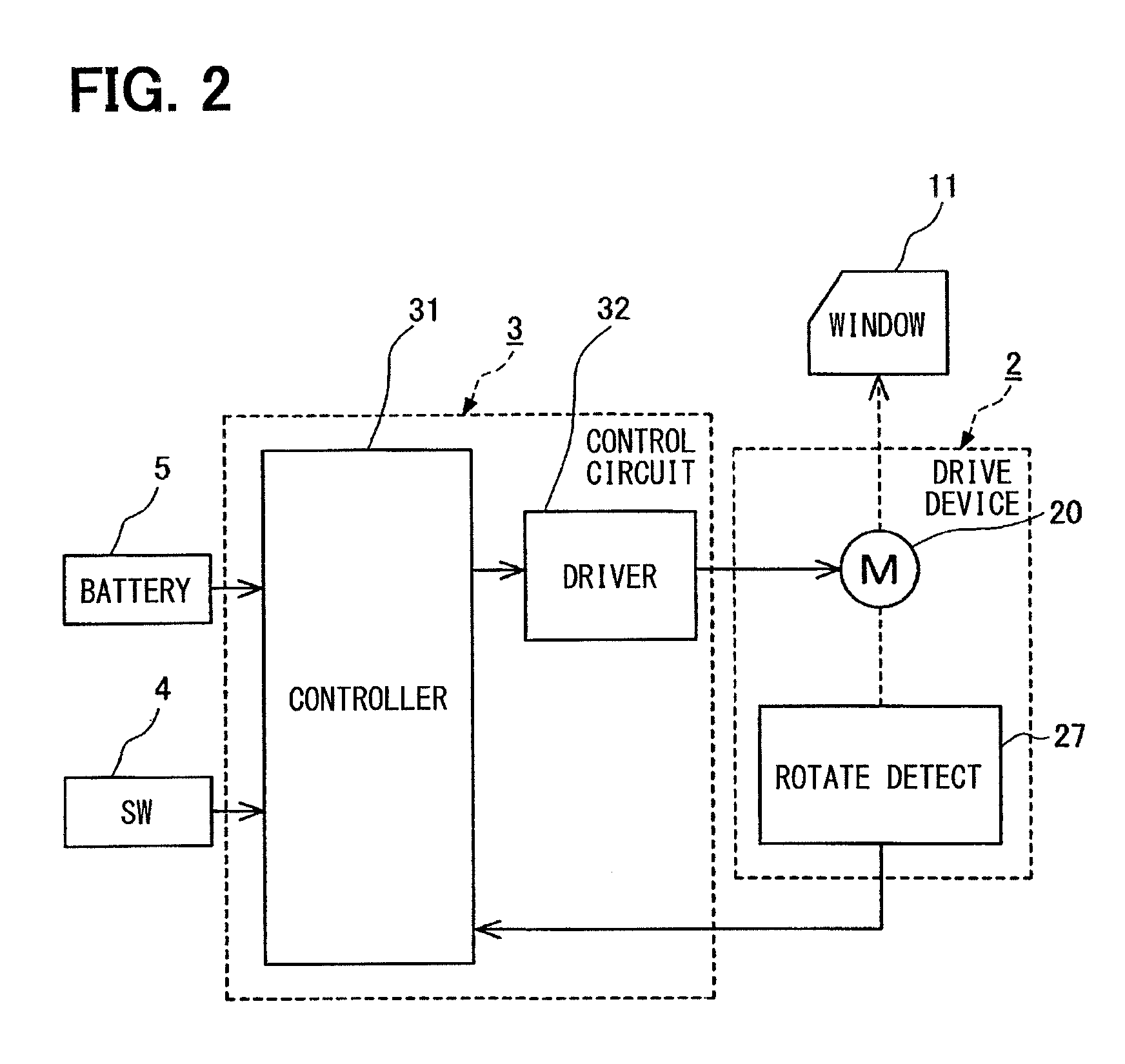
ActiveUS20140083011A1Stop progressErroneous operationPower-operated mechanismForeign matterElectric power system
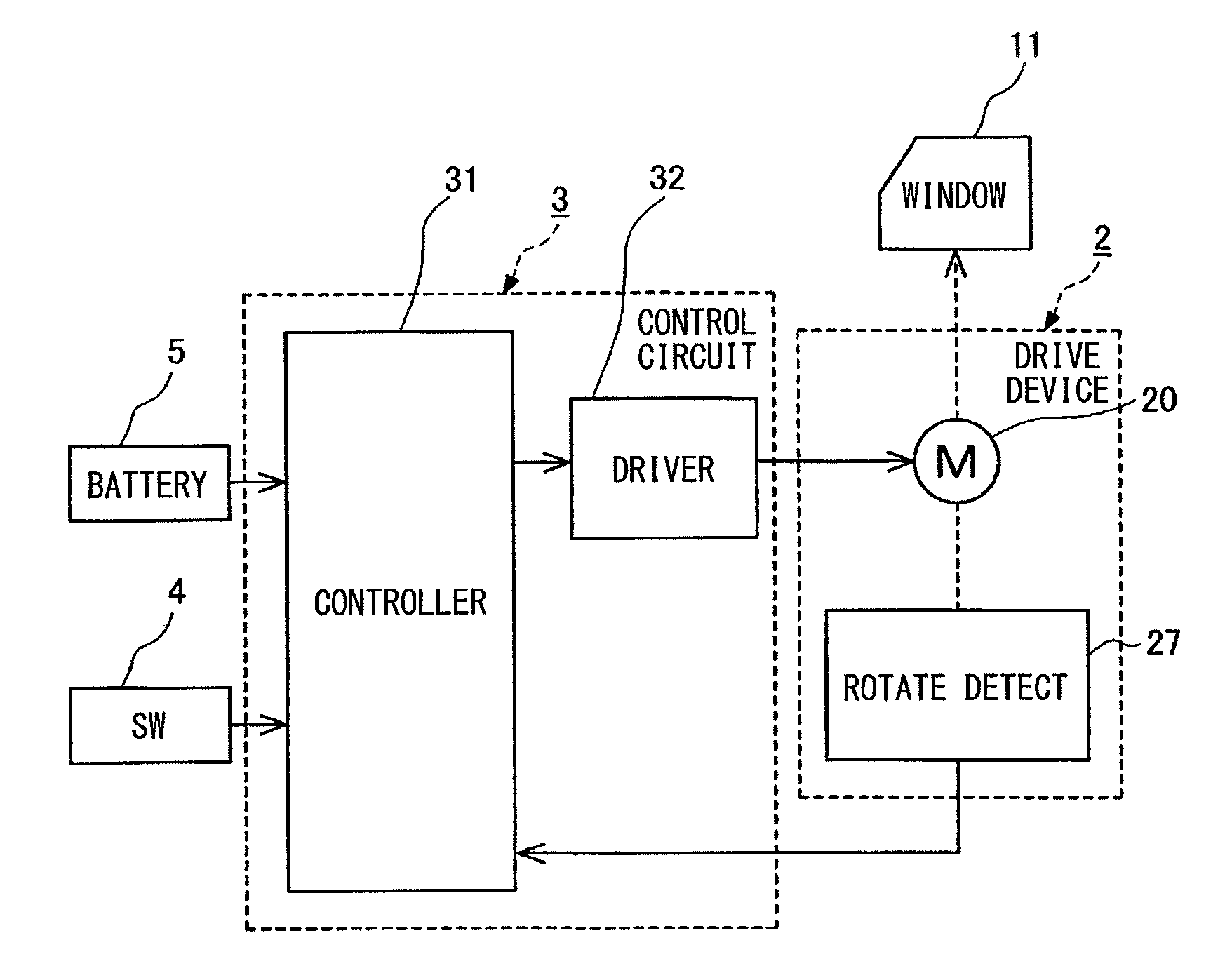
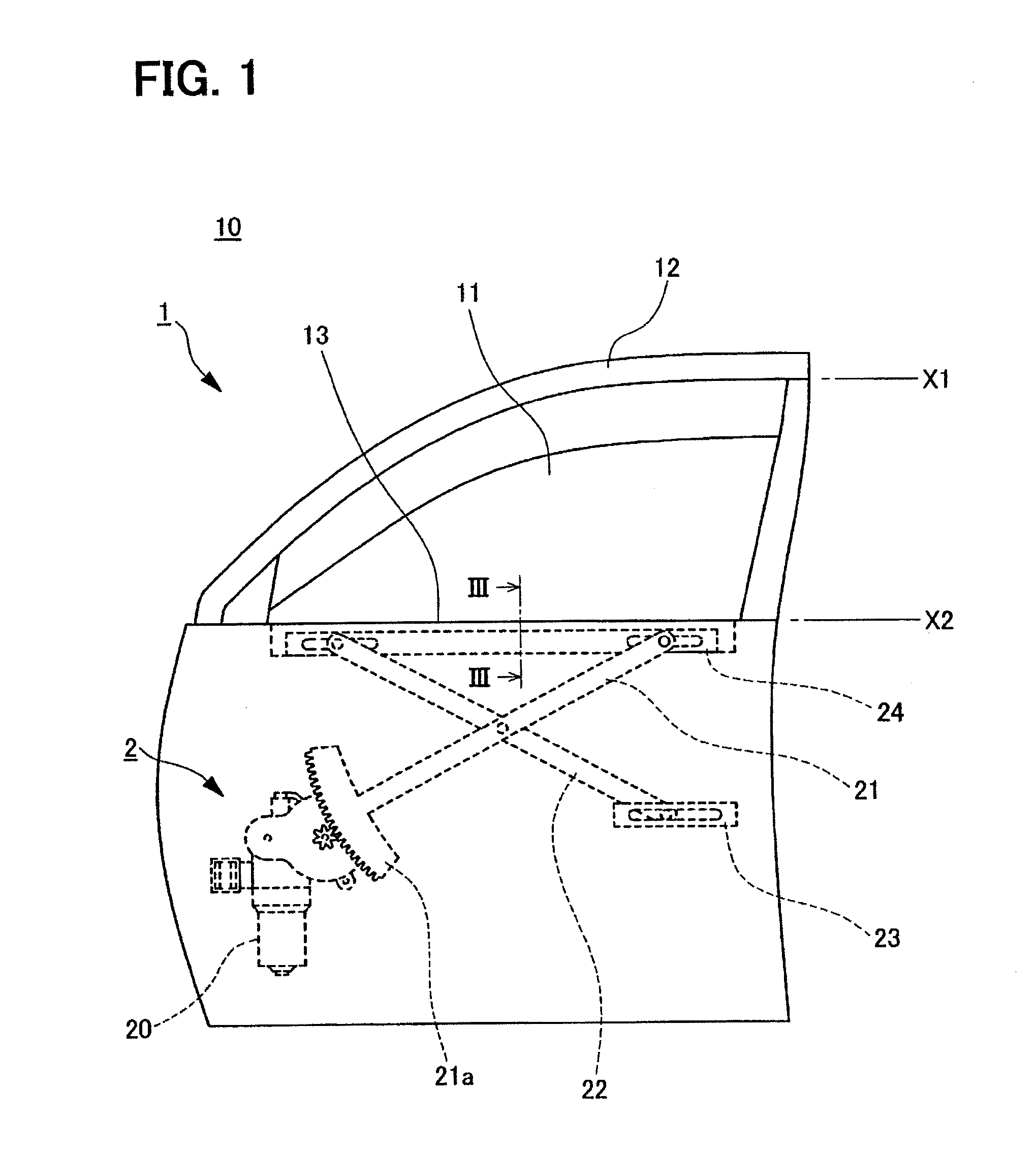
An open-close member control apparatus has a function to release a foreign matter pinched to an open-close member. The open-close member is either (i) driven only while a manipulation switch is manipulated or (ii) driven continuously once the manipulation switch is manipulated to a specified position regardless of whether the manipulation is then released. When trapping of a foreign matter is detected under an open movement of the open-close member to an open direction based on an open command signal from the manipulation switch, an electric power supply to a motor is restricted to stop a progress of the trapping. When receiving a close command signal from the manipulation switch after restricting the electric power supply, the electric power is supplied to the motor to drive the open-close member to a close direction under a restricted state of a predetermined operation.
Owner:DENSO CORP
Topical Treatment of Peripheral diabetic complications
InactiveUS20090018151A1Decreased microcirculationEffective treatmentOrganic active ingredientsBiocideSodium Channel InhibitorsNitric oxide
This invention relates to agents for treating, ameliorating, or preventing diabetic complications specifically diabetic neuropathy and skin ulceration by topical application of materials containing one or more arterial or venous dilation components and one or more fast sodium channel inhibitors. Particularly, the invention relates to treating or ameliorating agents that contain minoxidil and its related congeners, which have both potassium channel agonist properties and nitric oxide agonist properties as an active ingredient and which are effective against diabetic complications such as neuropathy, peripheral circulation disorders and skin ulcerations. Unique methods of delivery including delivery through impregnated socks or gloves to match the distribution of pain are also noted.
Owner:FINK EZEKIEL
Eye drops for cataract and its preparing method
InactiveCN1899304AGood treatment effectStop progressSenses disorderInorganic boron active ingredientsVitamin CSodium hyaluronate
The present invention relates to a kind of eye drops for cataract and its preparation process. It features the pH value of 6.0-8.0, and the osmotic pressure of 250-400 mOsm / Kg H2O, and consists of component A 100 ml and component B 1500-2400 mg, where the component A consists of taurine 600-1200 mg, sodium hyaluronate 100-500 mg, vitamin C 500-1500 mg, boric acid 500-1500 mg, borax 150-250 mg, sodium chloride 200-400 mg, EDTA disodium 100-150 mg, benzalkonium bromide 2-5 mg and water for injection for the rest in each 100 ml, and the component B is reducing glutathione. The eye drops are used in treating cataract in early stage and middle stage.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Friction stabilizer with tabs
InactiveUS20070196183A1Difficult to removeDecrease spaced distanceAnchoring boltsEngineeringSheet material
Owner:VALGORA GEORGE G
Use of soluble CD26 as inhibitor of angiogenesis and inflammation
InactiveUS20060051366A1Inhibit angiogenesisArresting of conditionPeptide/protein ingredientsAntibody ingredientsAngiogenesis growth factorBlood vessel
The present invention relates to soluble CD26 or a biologically active variant of the soluble CD26 that effectively inhibits angiogenesis and inflammation in a mammalian subject, pharmaceutical compositions comprising the soluble CD26 and the use of soluble CD26 in methods of treatment.
Owner:CHANG
Absorbable solid compositions for topical treatment of oral mucosal disorders
The invention provides a solid, self-bioadhesive composition for topical application that adheres to the oral mucosal tissue comprising a therapeutically effective amount of at least one herbal or homeopathic active agent; and a pharmaceutically acceptable solid bioadhesive carrier in an amount from about 40 to 99 percent based on the weight of the whole composition.
Owner:AXIOMEDIC
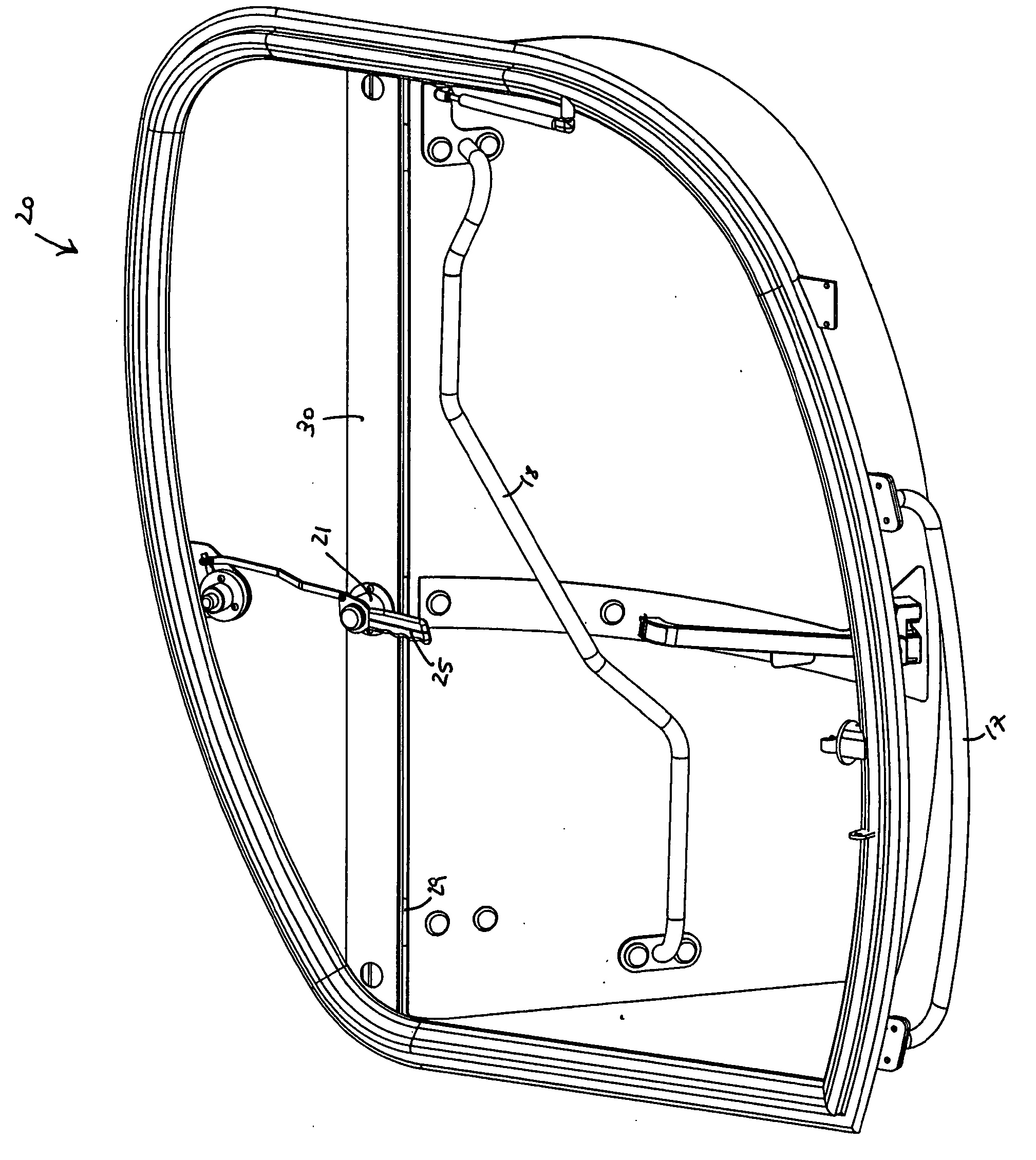
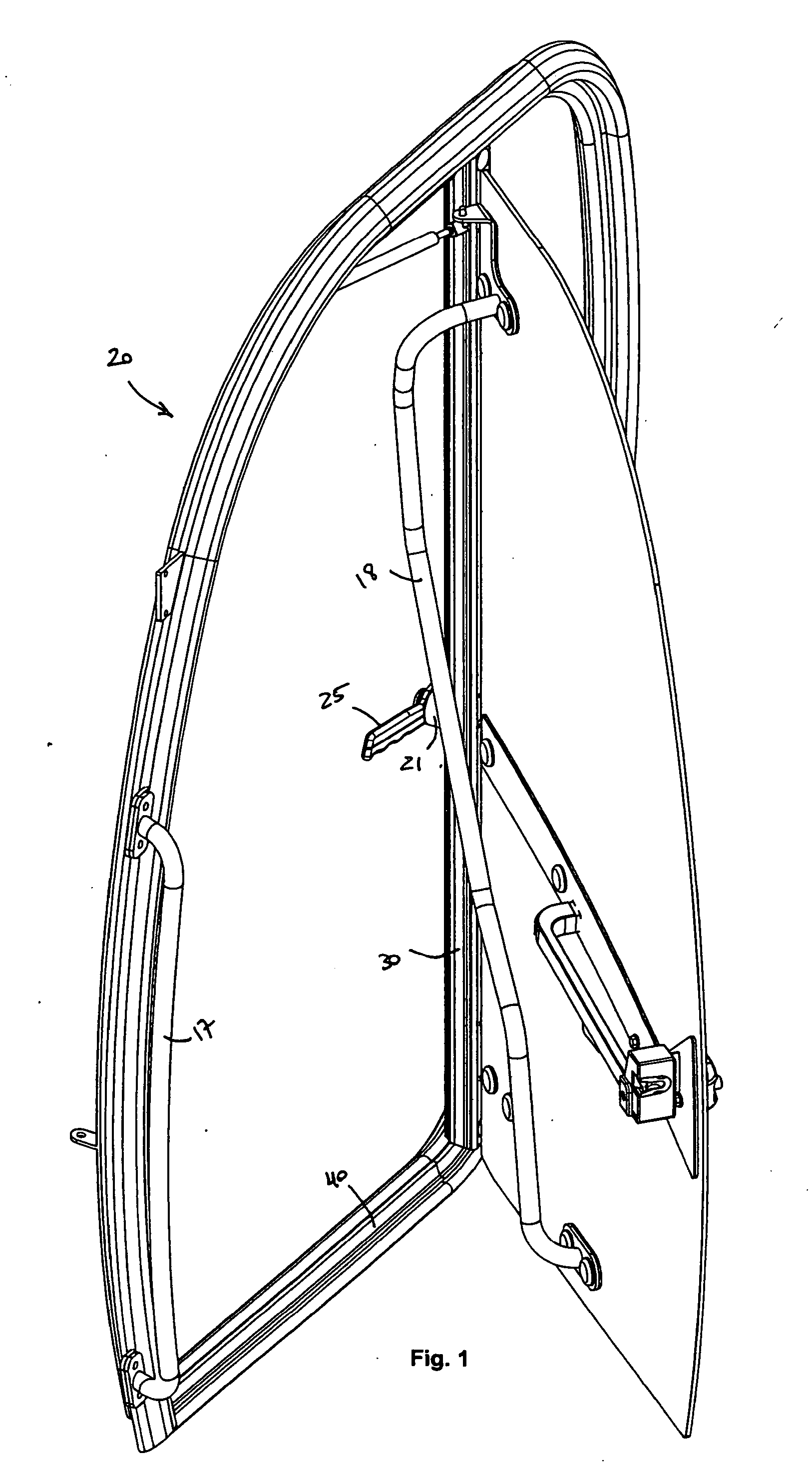
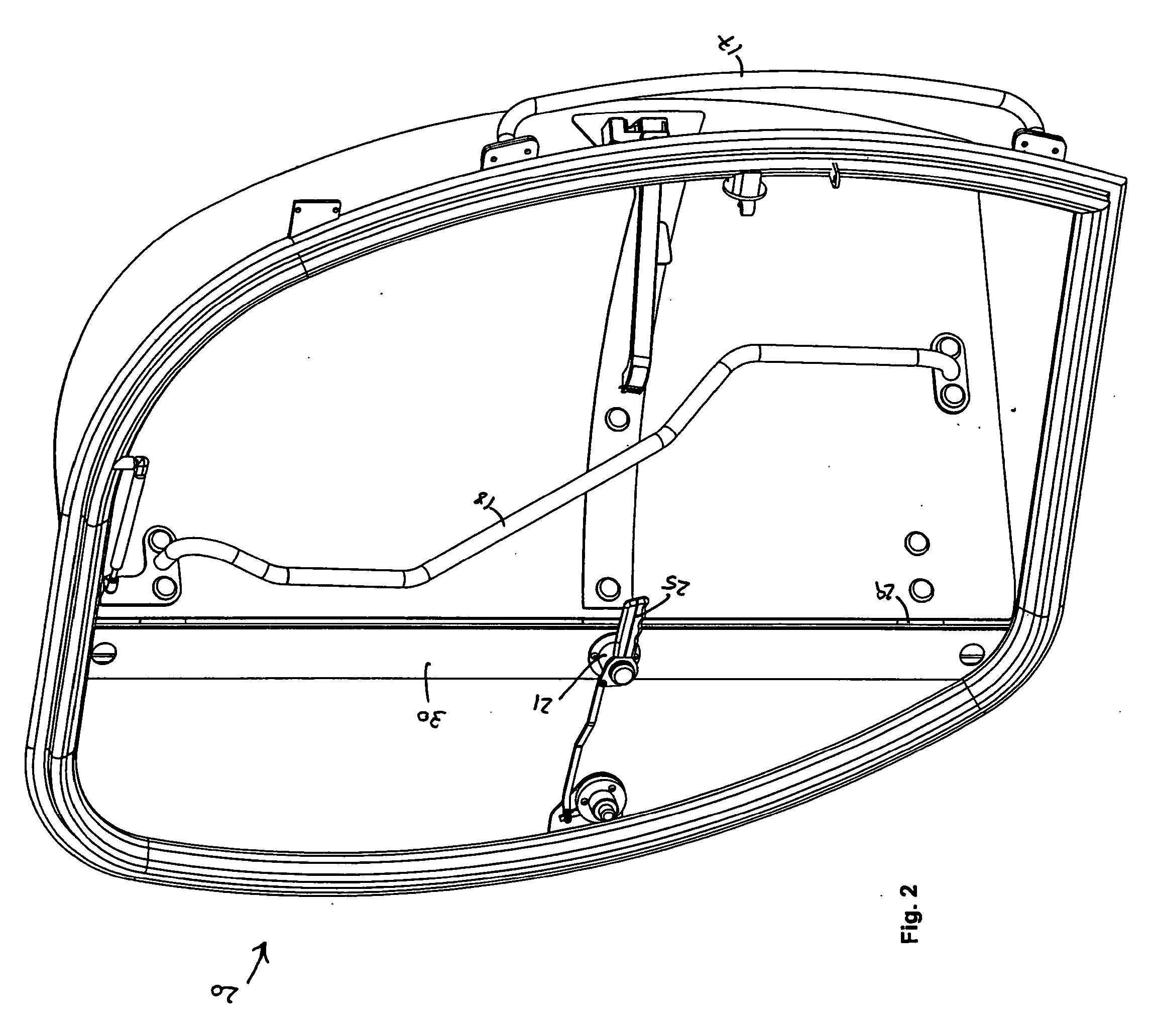
Dual Door Release Handle
InactiveUS20090033105A1Release fullyStop progressWing fastenersHingesMechanical engineeringEngineering
The present invention is a dual door release handle and related mechanisms for use with cabs of industrial vehicles that an operator may reach from outside of such a cab in order to fully release a door that has been propped open in order to close it. The dual door release handle of the present invention includes a latching mechanism having a handle mounted on the inside of the cab that extends into the doorway such that it may be reached through the open door of the cab by an operator who is located outside and / or below the cab. A linkage is provided between this handle and an operable device associated with the release mechanism inside the cab. The linkage is operatively attached to the handle and to the release mechanism such that turning the handle causes the linkage to move the release mechanism. Movement of the handle pulls the linkage and releases the holdback holding the open door against the side of the cab. Thus, an operator may reach up into the cab from the outside and pull down on the interior handle in order to release the door from its holdback position against the side of the cab allowing the door to swing freely so that it may be closed.
Owner:SAF T CAB
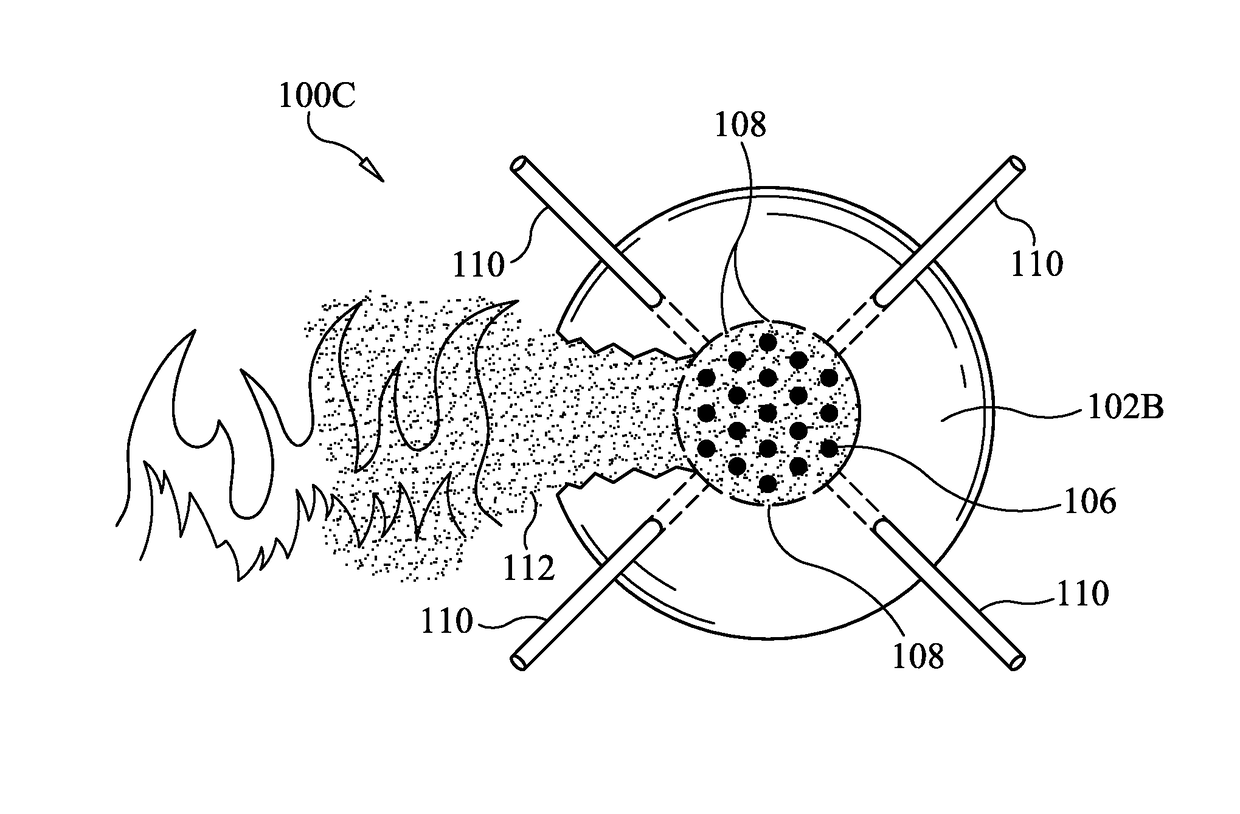
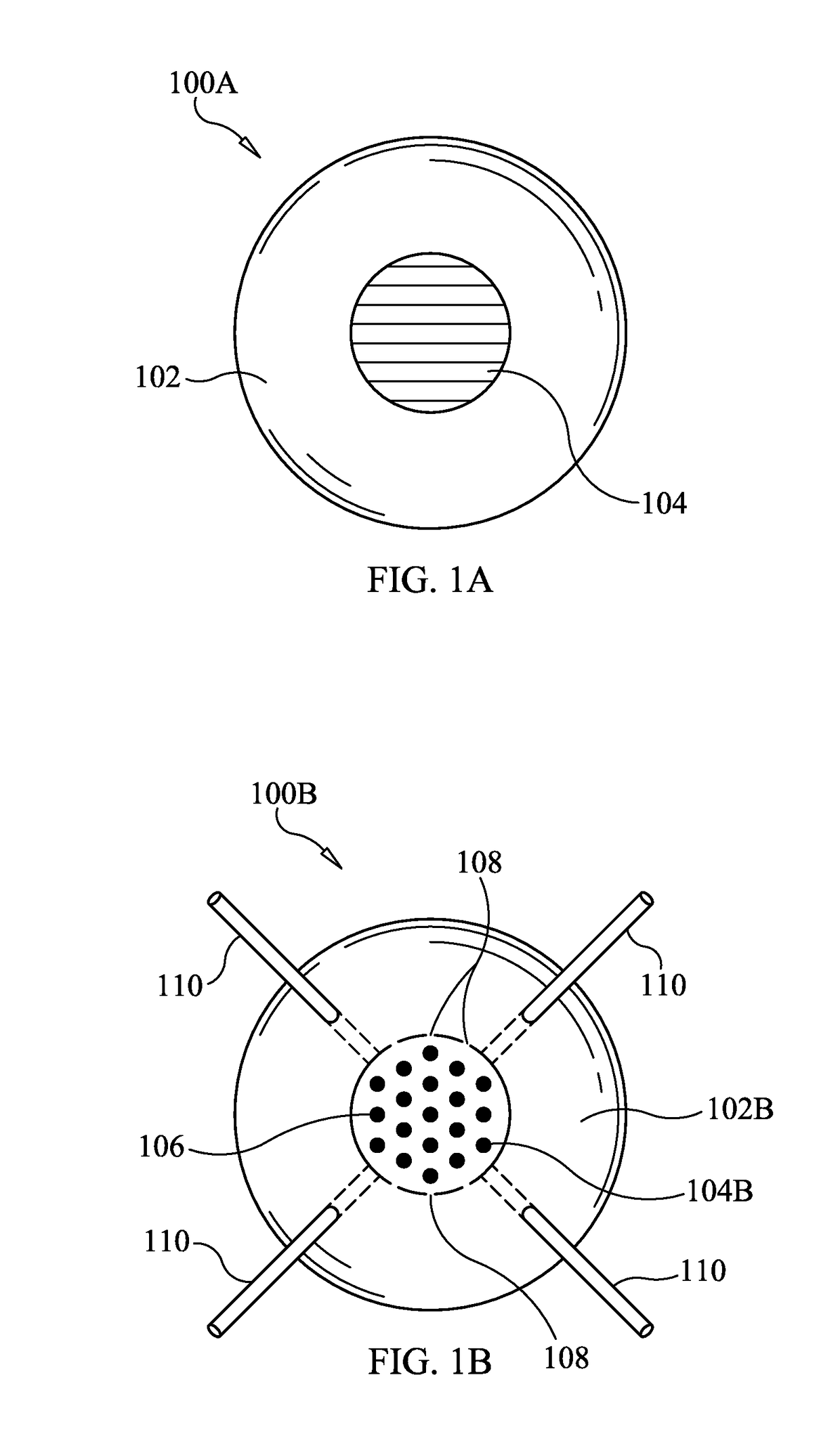
Fire suppression and/or extinguishment systems and devices
The present invention features systems, methods and devices directed to fire suppression and / or extinguishment using solid carbon dioxide and / or liquid or aqueous hydrogen. Systems and methods of deployment and / or dispersal of carbon dioxide and hydrogen (which would form water at its deployment location) are contemplated. The present invention also contemplates method(s) of fire suppression and / or extinguishment which may include the steps of: carbon dioxide canister deployment, formation of a carbon dioxide curtain and hydrogen deployment and / or dispersal.
Owner:GRAVITY WELLS TECH
Diagnostic Methods for Glaucoma
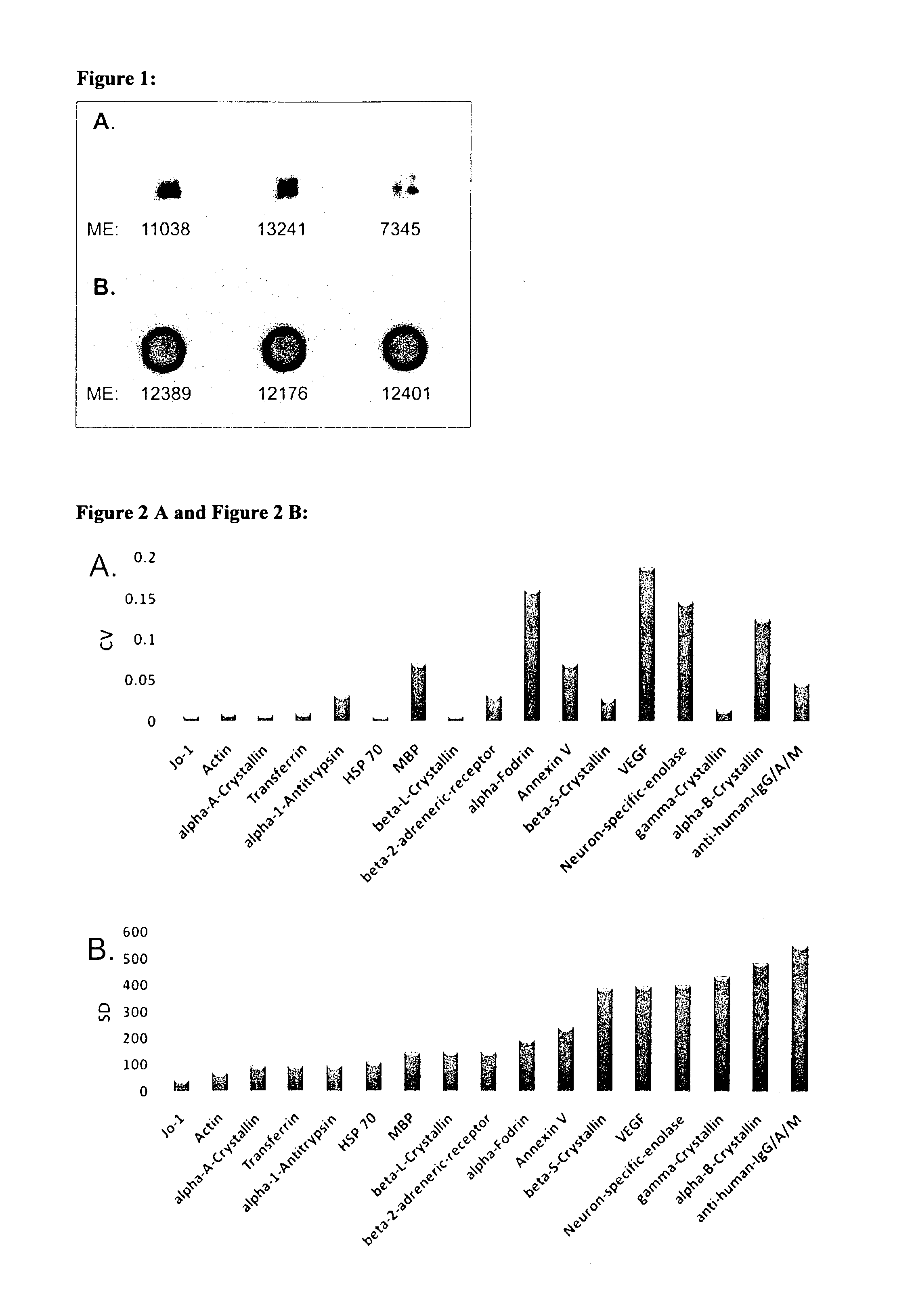
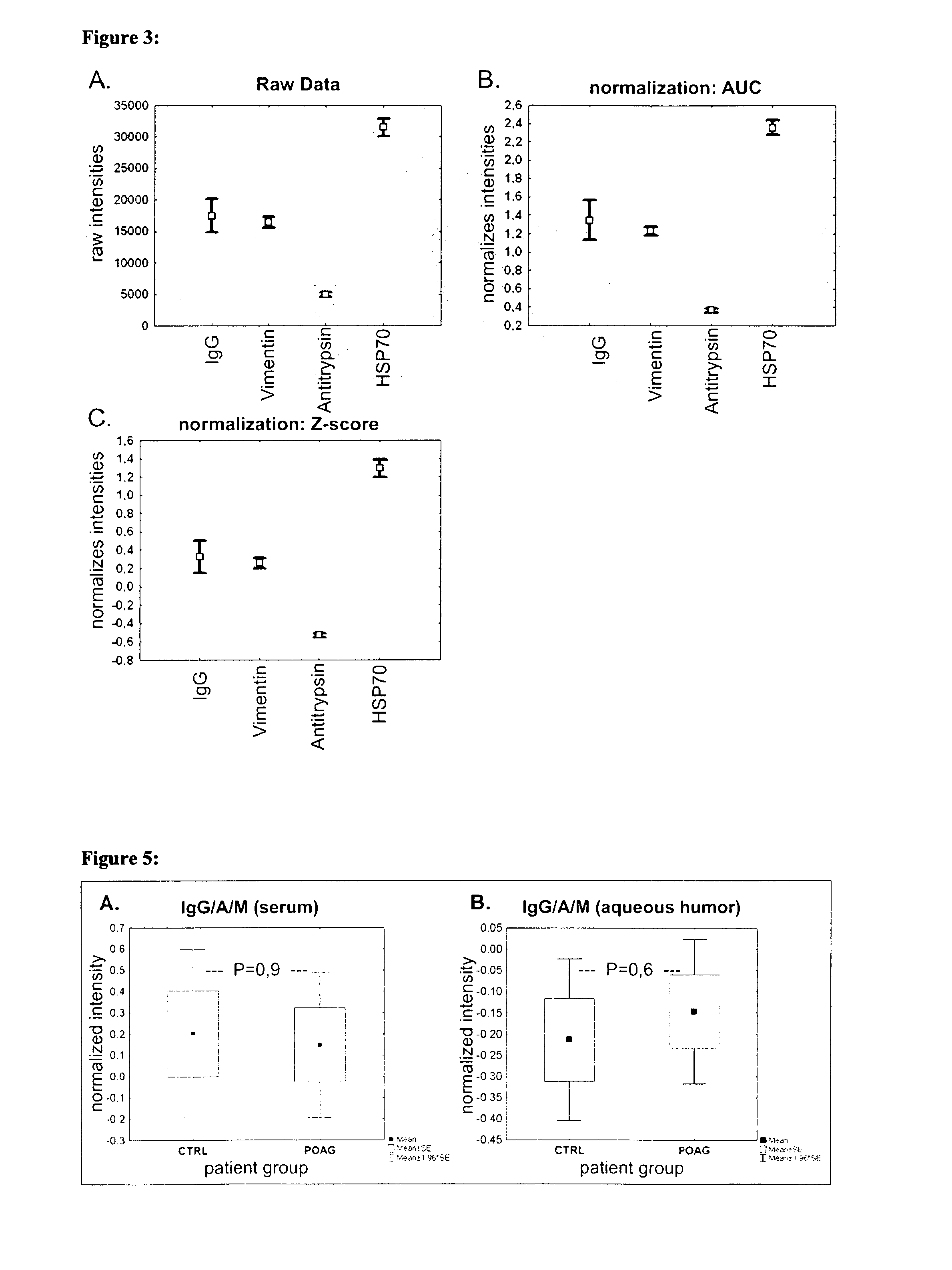
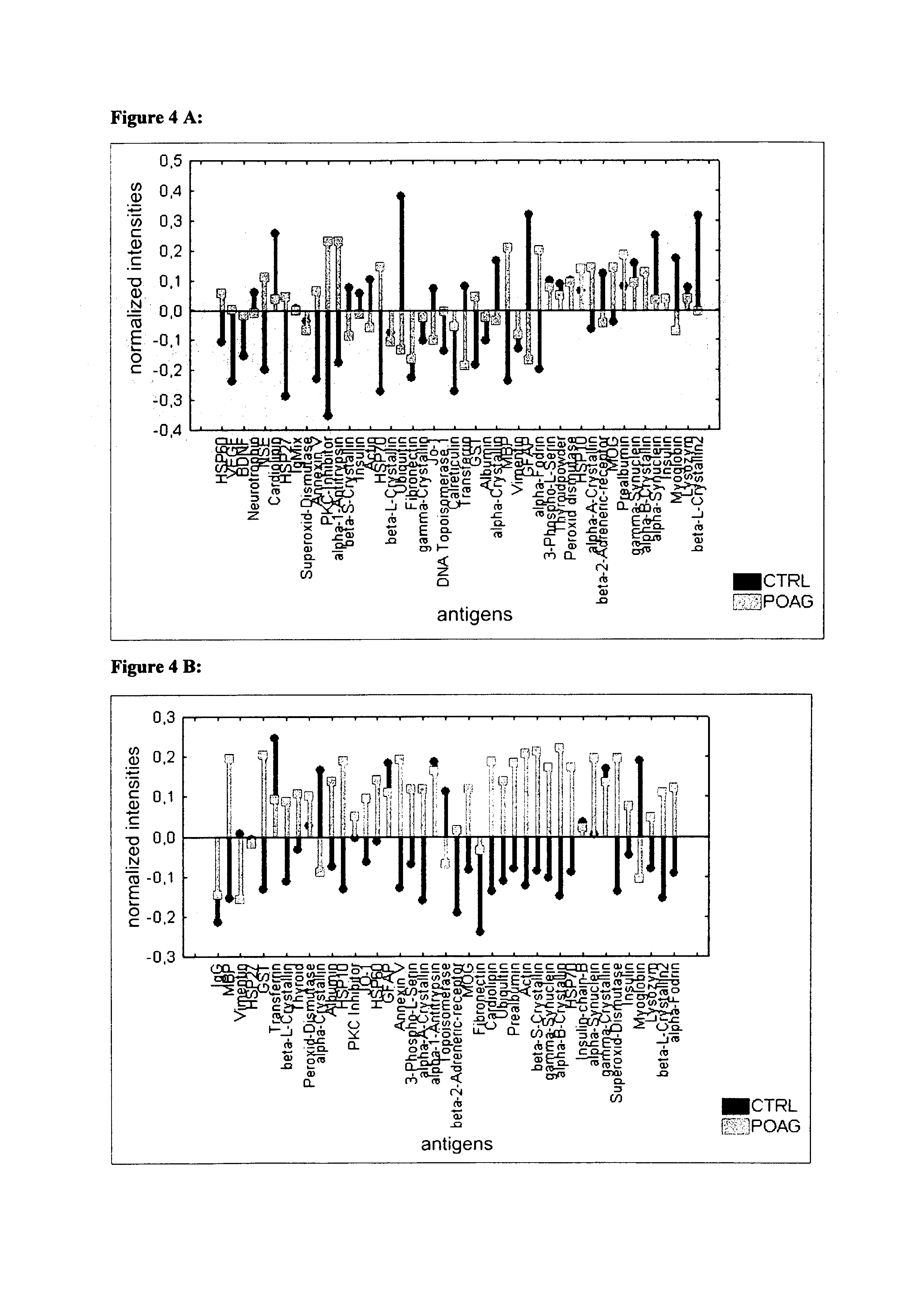
ActiveUS20130172204A1Avoid cell deathStop progressSenses disorderPeptide librariesAutoimmune ReactionsAfter treatment
The invention concerns a first diagnostic method for glaucoma based on an analysis of autoimmune reactivity in body fluids against at least one sample of at least partially purified ocular antigens, wherein the autoimmune reactivity against individual antigens is measured and transformed into a glaucoma score to determine the diagnostic result. Further aspects of the invention include antigen carrying elements carrying at least one sample of the at least partially purified ocular antigens and kits for diagnosis of glaucoma. Further aspects include methods of collecting a body fluid such as tears for the use in the diagnostic method for glaucoma. Yet further aspects include ocular antigens serving as diagnostic markers and / or for preparing pharmaceutical compositions for treatment of glaucoma. The invention further concerns a second diagnostic method for glaucoma comprising the steps of a) providing an in vitro culture of cells; b) incubating a body fluid from a test individual with the in vitro culture of cells or incubating components, which are fractionated from the body fluid or from a body specimen of the test individual with the in vitro culture of cells; c) analyzing protein expression of the cells and / or analyzing the viability of the cells after treatment according to step b); and d) comparing the results of the analysis in step c) with standard data to determine a diagnostic result.
Owner:M LAB GMBH
Absorbable solid compositions for topical treatment of oral mucosal disorders
InactiveUS20110244039A1Stop progressEffective treatmentAntibacterial agentsBiocideDiseaseMucosal disease
The invention provides a solid, self-bioadhesive composition for topical application that adheres to the oral mucosal tissue comprising a therapeutically effective amount of at least one herbal or homeopathic active agent; and a pharmaceutically acceptable solid bioadhesive carrier in an amount from about 40 to 99 percent based on the weight of the whole composition.
Owner:AXIOMEDIC
Safe botanical drug for treating malignant pleural effusion and cancer and increasing immune function
InactiveUS20030152647A1Specific activityInhibit oncogeneBiocideUnknown materialsAbnormal tissue growthAnticarcinogen
Three new safe pharmaceutical compositions in accordance with the present invention are for treatment and prevention of malignant pleural effusion and increase immune function comprise Lan Xiang Xi (LX) and polysaccharide of Dang Gui. Methods of treating and preventing cancer cells include inhibiting oncogenes, increasing activity of tumor suppressor, inducing differentiation of cancer cells, inhibiting cancer cells proliferation, inducing apoptosis of cancer cells, inhibiting growth of transplanted cancer and inhibiting cancer incidence, etc. In general, anticancer drug always decreases immune function. However, LX and LX+PDG can inhibit cancer also increase immune function at the same time. Also, LX and DG containing soybean-liposomes is safer than LX and DG.
Owner:LIU YAGUANG
Gene therapy compositions for preventing and/or treating of autoimmune diseases
InactiveUS20140045923A1Avoid developmentAvoid lostPeptide/protein ingredientsMetabolism disorderInsulin-like growth factorAutoimmune thyroid disease
Gene therapy compositions for the prevention and / or treatment of autoimmune diseases. The present invention relates to vectors, preferably plasmids that comprise specific promoters, that encode insulin-like growth factor I (IGF-I) or a functional biological equivalent thereof, which will be used to prevent or treat Type 1 Diabetes (DT1) and / or other autoimmune diseases. Moreover, the invention comprises the method of preventing or treating DT1 by means of the expression of IGF-I, or a functional biological equivalent thereof, in hepatic cells. IGF-I was capable of interrupting the immune attack against pancreatic β cells, thereby preventing the loss of β cell mass and, consequently, maintaining normal levels of circulating insulin.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA
One-handed fire starter
A novel mechanical spark generating device for one-handed operation. The novel mechanical spark generating device includes a case having a case body and a relatively moveable striker arm extended therefrom, the case body includes a channel having an opening thereinto. A pyrophoric rod assembly is moveably carried in the channel of the case body, the pyrophoric rod assembly having a support surface adjacent to one end thereof, and a pyrophoric rod carried therein. A striker bar is fixed to the striker arm in a position for interacting with the pyrophoric rod of the pyrophoric rod assembly when the pyrophoric rod assembly is in a deployed configuration extended from the case body and the striker arm is moved there toward.
Owner:ESSENTIAL GEAR
Method for improving anticancer activity of Codonopsis lanceolata Benth.et Hook.F
InactiveCN102091117AStop the progression of malignancyStop progressAntineoplastic agentsPlant ingredientsYeastCodonopsis pilosula
The invention relates to a method for improving the anticancer activity for Codonopsis lanceolata Benth.et Hook.F, which comprises: slicing or homogenizing fresh Codonopsis lanceolata Benth.et Hook.F, or crushing dry Codonopsis lanceolata Benth.et Hook.F, or powdering the dry Codonopsis Lanceolata Benth.et Hook.F; soaking in water; sterilizing under a high pressure; adding 0.5 to 5.0 percent of active yeast and 1 to 8 percent of glucose under a sterile condition when the temperature lowers to 30 DEG C; fermenting at 20 to 37 DEG C for 1 to 10 days, and adding a polar solvent after fermentation to extract for 5 to 24 hours at 0 to 100 DEG C; and filtering, recovering solvent and concentrating under reduced pressure or drying by freezing. The method can improve the inhibition effect of Codonopsis lanceolata Benth.et Hook.F on the growth of tumors.
Owner:YANBIAN UNIV
Trailer restraint with auxiliary securing/locking mechanism
The present invention is an impact vehicle restraint with a hook and auxiliary securing / locking mechanism that combine to secure the RIG bar of a trailer to a loading dock. A motor rotates the hook between retracted and extended positions. When extended, the hook secures and holds the RIG bar to the dock. When the hook is extended, the auxiliary securing / locking mechanism is also extended to and held at a set position by a biasing mechanism. When the hook is blocked by an obstruction, forward movement of the trailer causes the RIG bar and the obstruction to slide into engagement with the auxiliary securing / locking mechanism, which accepts the RIG bar and obstruction and moves to a locked position to secure the RIG bar. The retraction of the hook by the motor overpowers the biasing mechanism and causes the retraction of the auxiliary securing / locking mechanism.
Owner:NORDOCK
Composition for ocular topical administration treatment ocular hypertension and glaucoma
InactiveUS20120232139A1Reduce the amount requiredLower eye pressureOrganic active ingredientsBiocideSide effectBenzalkonium chloride
The present invention provides a composition for ocular topical administration for treating ocular hypertension and glaucoma, comprising latanoprost as an active ingredient, and (a) a polyol and / or sugar alcohol, (b) a nonionic surface active agent, and (c) an edetic acid compound. The composition of the present invention comprises lower amount of preservatives such as benzalkonium chloride comparative to the conventional product and therefore, can reduce incidence of the adverse side effects caused by the preservatives. In addition, the composition of the present invention can be stored stably at room temperatures for a long term.
Owner:SUCAMPO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com