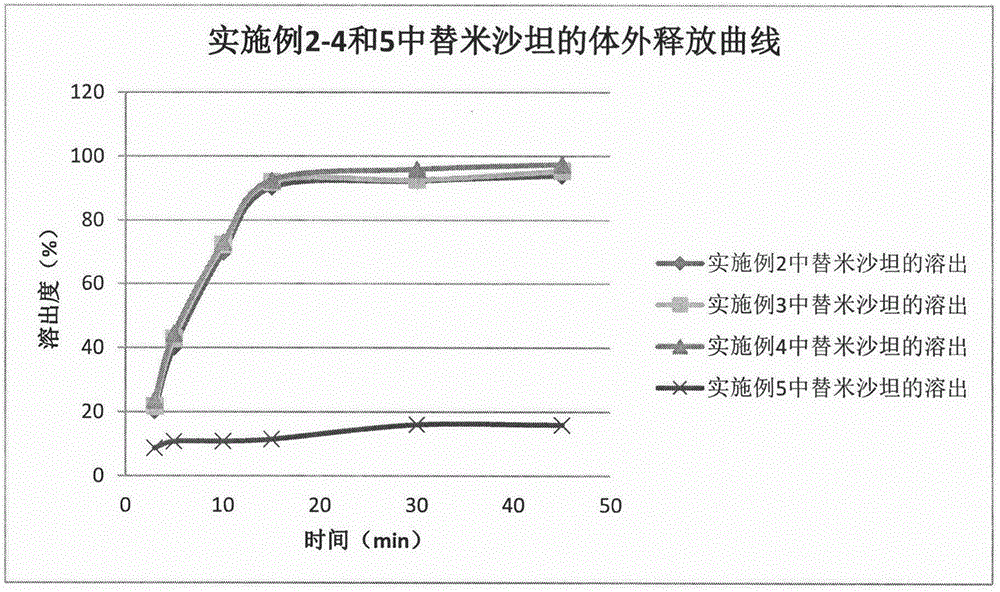
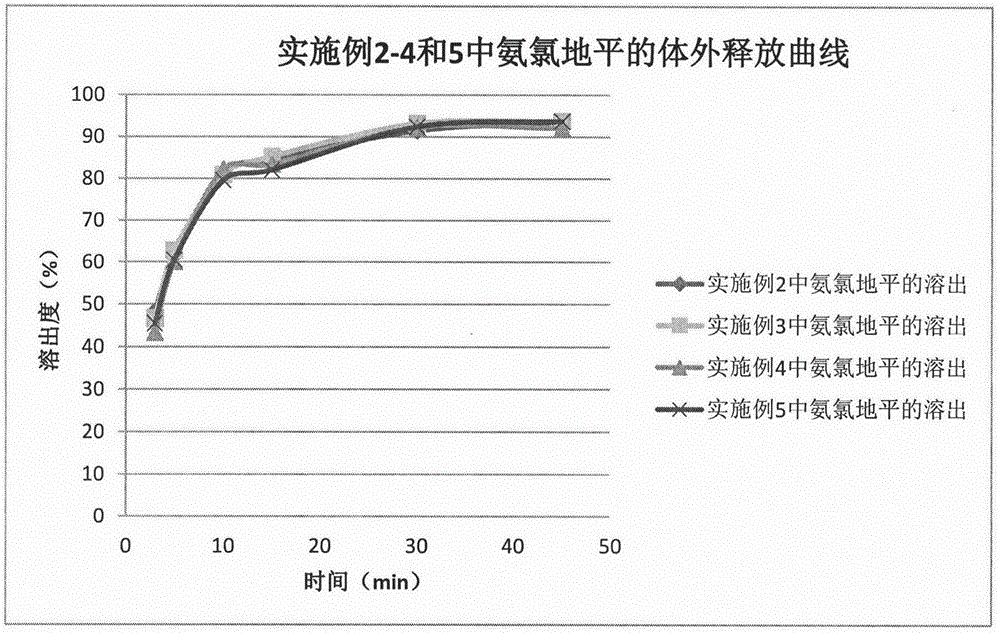
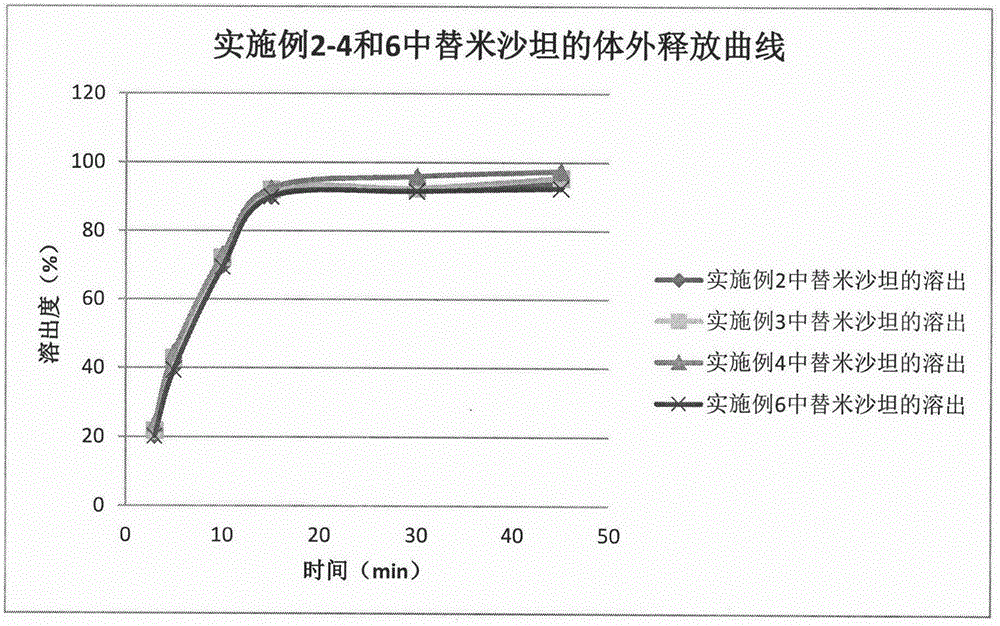
Method for preparing telmisartan and amlodipine double-layer tablets
A technology of amlodipine besylate layer and telmisartan, which is applied in directions such as medical preparations without active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve the problem of the stability of amlodipine besylate. Influence and other issues, to achieve good dissolution behavior, ensure quality, and ensure the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: the screening of solid dispersion carrier Eudragit E100 dosage
[0056] The composition of the prescription of a solid dispersion containing Telmisartan and Eudragit E100 in different proportions is as follows:
[0057]
[0058] The preparation process of the solid dispersion: According to the prescription amount, add the telmisartan raw material drug into ethanol, stir and dissolve until the solution is clear, then add Eudragit E100, continue to stir and dissolve until the solution is clear. Then, under the condition that the temperature does not exceed 40°C, vacuumize, quickly distill off the organic solvent under reduced pressure, dry under reduced pressure overnight, and pass through a 40-mesh sieve after crushing to obtain a solid dispersion of telmisartan, which is sealed and stored for future use.
[0059] Solubility test: Weigh a certain amount of telmisartan solid dispersion and telmisartan raw material prepared by the above prescriptions 1 to ...
Embodiment 2
[0062] Embodiment 2: the preparation of telmisartan amlodipine bilayer tablet
[0063] The prescription of telmisartan and amlodipine double-layer tablet is composed as follows (making 1000 tablets altogether):
[0064]
[0065] The preparation process of telmisartan amlodipine bilayer tablet is as follows:
[0066] Add Telmisartan into ethanol according to the prescribed amount, stir and dissolve until the solution is clear, then add Eudragit E100, continue stirring and dissolving until the solution is clear. Under the condition that the temperature does not exceed 40°C, vacuumize, quickly evaporate the organic solvent under reduced pressure, dry under reduced pressure overnight, pass through a 40-mesh sieve after crushing, and then fully mix with sorbitol and microcrystalline cellulose, then add stearic acid After the magnesium is fully mixed again, the mixed granules of the telmisartan layer are obtained, and the content of the mixed granules is detected for subsequent ...
Embodiment 3
[0069] Embodiment 3: the preparation of telmisartan amlodipine bilayer tablet
[0070] The prescription of telmisartan and amlodipine double-layer tablet is composed as follows (making 1000 tablets altogether):
[0071]
[0072] The preparation process of telmisartan amlodipine bilayer tablet is as follows:
[0073] Add Telmisartan into ethanol according to the prescribed amount, stir and dissolve until the solution is clear, then add Eudragit E100, continue stirring and dissolving until the solution is clear. Under the condition that the temperature does not exceed 40°C, vacuumize, quickly evaporate the organic solvent under reduced pressure, dry under reduced pressure overnight, pass through a 40-mesh sieve after crushing, and then fully mix with sorbitol and microcrystalline cellulose, then add stearic acid After the magnesium is fully mixed again, the mixed granules of the telmisartan layer are obtained, and the content of the mixed granules is detected for subsequent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com