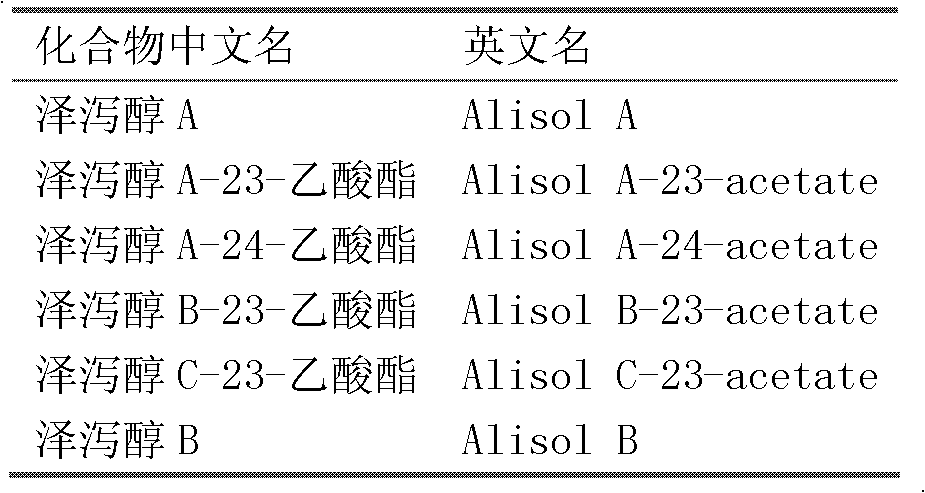
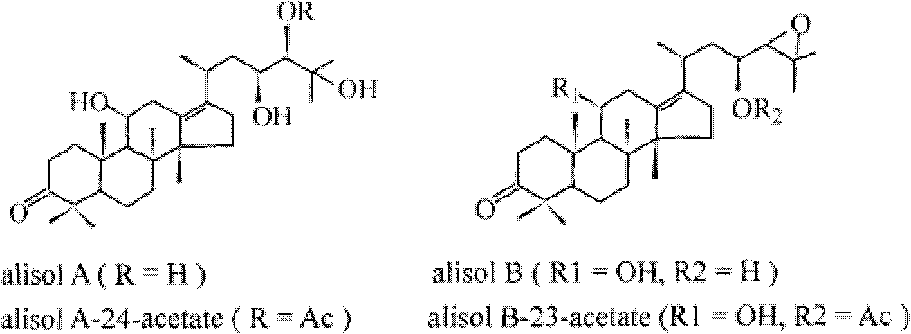
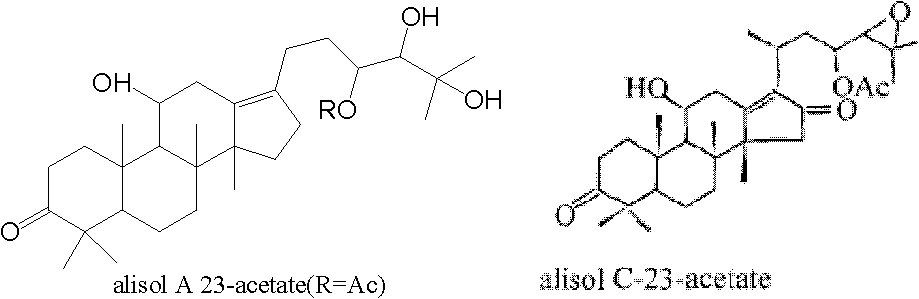
Composition containing alisol A and application of composition containing alisol A on medicine
A technology of alisol and its composition, which is applied in the field of compositions containing alisol A, can solve the problems that the quality stability is not good enough, and the aortic inner wall thickness and inflammatory indicators of rabbits with atherosclerosis cannot be significantly improved, etc. , to achieve the effects of long-term use, increased stability, and less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Effects of different compositions on the stability of alisol A-23-acetate
[0028] Stability of the compound alisol A-23-acetate when alisol A and alisol A-24-acetate co-exist in solution.
[0029] Take the following samples, dissolve them in 1000ml of 50% ethanol solution, ultrasonically dissolve, measure the concentration of alisitol A-23-acetate in the solution by HPLC, heat in a water bath at 60°C, and measure the concentration of alisol A-23-acetate in the solution at 1h, 2h and 5h respectively. Concentrations of alisol A-23-acetate. Each sample was run in 3 parallels.
[0030] No. 1 Alisitol A-23-Acetate 100mg
[0031] No.2 Alisitol A+Alisol A-23-Acetate+Alisol A-24-Acetate, 1:0.05:0.5, 3100mg
[0032] No.3 Alisitol A+Alisol A-23-Acetate+Alisol A-24-Acetate, 1:0.3:0.05, 450mg
[0033] No.4 Alisitol A+Alisol A-23-Acetate+Alisol A-24-Acetate, 1:0.1:0.2, 1300mg
[0034] No. 5 alisol A + alisol A-23-acetate + alisol A-24-acetate, 1:0.05:0.05, 2200mg
[0035] No....
Embodiment 2
[0044] Comparison of lipid-lowering effects of different compositions on hyperlipidemic rats
[0045] Get the SD male rats of body weight 190-210g, 222, get 10 at random and give conventional feed as blank control group, all the other 210 give high-fat feed (cholesterol 1%, lard 20%, bile salt 0.2%, conventional feed 78.8%), the serum total cholesterol (TC), serum triglyceride (TG) and low-density lipoprotein (LDL-C) of each rat fed with high-fat diet were measured after 2 weeks. According to the elevated values of TC, TG and LDL-C, the top 120 rats with elevated TC, TG and LDL-C were selected and randomly divided into 12 groups, with 10 rats in each group, respectively:
[0046] Model control group (purified water),
[0047] Simvastatin group (Harbin Pharmaceutical Group Sanjing Pharmaceutical Co., Ltd., batch number: 0608206, 10mg / tablet, 2mg / kg)
[0048] Fenofibrate group (Shanghai Hengshan Pharmaceutical Co., Ltd., batch number: 071001, 100mg / tablet, 80mg / kg)
[0049]...
Embodiment 3
[0087] Effects of Different Compositions of A on Lowering Blood Lipid and Anti-Atherosclerosis in Rabbits with Atherosclerosis
[0088] 3.1 Dose and grouping
[0089] Animal species: Japanese big-eared white rabbit.
[0090] Animal level: normal level.
[0091] Sex and number of animals: 180, male.
[0092] Animal age: 5-6 months old.
[0093] Animal weight: 2-3kg.
[0094] Animals are grouped into:
[0095] Blank control group (purified water)
[0096] Model control group (purified water),
[0097] Simvastatin group (1.5mg / kg)
[0098] Fenofibrate group (60mg / kg)
[0099] Group M (Alisol A, 60mg / kg)
[0100] Group N (Alisol A+Alisol A-23-acetate, 1:1, 60mg / kg)
[0101] Group O (Alisol A+Alisol A-24-acetate, 1:1, 60mg / kg)
[0102] Group P (Alisol A+Alisol A-23-acetate+Alisol A-24-acetate, 1:0.05:0.5, 60mg / kg)
[0103]Group Q (Alisol A+Alisol A-23-acetate+Alisol A-24-acetate, 1:0.3:0.05, 60mg / kg)
[0104] Group R (Alisol A+Alisol A-23-acetate+Alisol A-24-acetate, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com