Medicine composition containing magnesium isoglycyrrhizinate and preparation method
A technology of magnesium isoglycyrrhizinate and magnesium glycyrrhizinate tablet cores, applied in the field of medicine, can solve the problems of poor oral bioavailability, unstable magnesium isoglycyrrhizinate, etc., and achieve the effects of convenient medication, improved dissolution, and improved in vivo bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
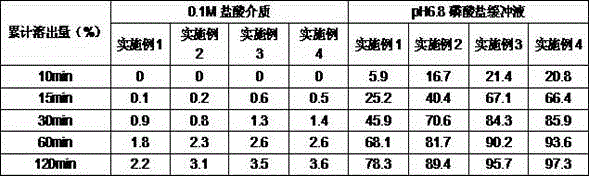
Examples
Embodiment 1
[0036] Example 1 Magnesium Isoglycyrrhizinate Enteric-Coated Tablets (10000 Tablets) Prescription and Preparation Method
[0037] Tablet core prescription
[0038] The name and amount of raw and auxiliary materials (kg)
[0039] Magnesium Isoglycyrrhizinate 3.0
[0040] Microcrystalline Cellulose 1.5
[0041] Mannitol 1.5
[0042] Povidone K300.24
[0043] Crospovidone 0.18
[0044] Magnesium Stearate 0.10
[0045] Enteric coating prescription
[0046] The name and amount of raw and auxiliary materials (kg)
[0047] Acrylic resin Ⅱ 0.3
[0048] Triethyl citrate 0.5
[0050] 1. Preparation process of magnesium isoglycyrrhizinate tablet core
[0051] 1) Grind magnesium isoglycyrrhizinate, microcrystalline cellulose, povidone K30, crospovidone, and magnesium stearate through a 100-mesh sieve respectively, and weigh the prescribed amount of magnesium isoglycyrrhizinate, microcrystalline cellulose, mannitol, and povidone K30, crospovidone, and m...
Embodiment 2
[0056] Example 2 Magnesium Isoglycyrrhizinate Enteric-Coated Tablets (10000 Tablets) Prescription and Preparation Method
[0057] Tablet core prescription
[0058] The name and amount of raw and auxiliary materials (kg)
[0059] Magnesium Isoglycyrrhizinate 3.0
[0060] Microcrystalline Cellulose 1.5
[0061] Mannitol 1.5
[0062] Povidone K300.24
[0063] Crospovidone 0.18
[0064] Magnesium Stearate 0.10
[0065] Enteric coating prescription
[0066] The name and amount of raw and auxiliary materials (kg)
[0067] Acrylic resin Ⅱ 0.3
[0068] Triethyl citrate 0.5
[0070] Dissolution layer prescription
[0071] The name and amount of raw and auxiliary materials (kg)
[0072] Hydroxypropyl methylcellulose 0.075
[0074] 1. Preparation process of magnesium isoglycyrrhizinate tablet core
[0075] 1) Grind magnesium isoglycyrrhizinate, microcrystalline cellulose, povidone K30, crospovidone, and magnesium stearate t...
Embodiment 3
[0083] Example 3 Magnesium Isoglycyrrhizinate Enteric-Coated Tablets (10000 Tablets) Prescription and Preparation Method
[0084] Tablet core prescription
[0085] The name and amount of raw and auxiliary materials (kg)
[0086] Magnesium Isoglycyrrhizinate 3.0
[0087] Microcrystalline Cellulose 1.5
[0088] Mannitol 1.5
[0089] Povidone K300.24
[0090] Crospovidone 0.18
[0091] Magnesium Stearate 0.10
[0092] Enteric coating prescription
[0093] The name and amount of raw and auxiliary materials (kg)
[0094] Acrylic resin Ⅱ 0.3
[0095] Triethyl citrate 0.5
[0096] Talc powder 0.3
[0097] Dissolution layer prescription
[0098] The name and amount of raw and auxiliary materials (kg)
[0099] Hydroxypropyl methylcellulose 0.075
[0100] Talc powder 0.1
[0101] 1. Preparation process of magnesium isoglycyrrhizinate tablet core
[0102] 1) Airflow crushing magnesium isoglycyrrhizinate, particle size D50≤5μm, D90≤10μm.
[0103] 2) Weigh the prescribed am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com