Anticoagulant immediate-release pharmaceutical preparation and preparation method thereof
A technology for pharmaceutical preparations and anticoagulants, which is applied in the field of medicine, can solve the problems such as the prescription process report of a single active ingredient preparation without vecagrel, and achieve high in vivo bioavailability and blood drug concentration, large drug absorption degree, and stability. excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 vicagrel capsule
[0059]
[0060]
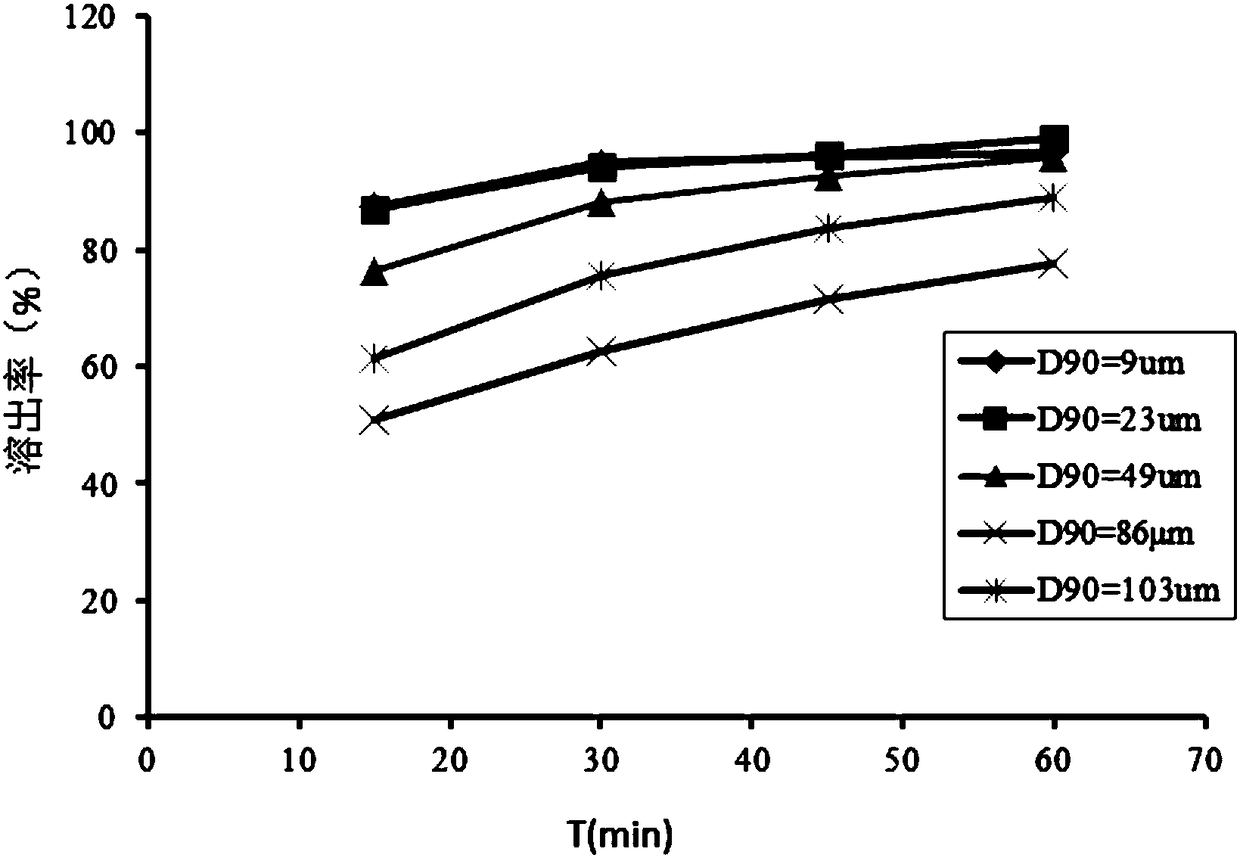
[0061] Vicagrelor was pulverized with a hammer mill (Frewitt) with a 0.20 mm screen, a rotating speed of 6000 rpm, and a feed rate of 1 kg / min, and the measured D90 = 43 μm. Mix the pulverized vicagrel with microcrystalline cellulose and lactose in a three-dimensional mixer for 15 minutes, add hydrogenated castor oil and mix, and fill the obtained granules into No. 3 capsules.
Embodiment 2
[0062] Embodiment 2 vicagrel capsules
[0063] Raw materials
[0064] Use QL-100 jet mill to pulverize the raw material, the pressure is 0.8MPa, the working temperature is 15°C, the pulverization time is 10min, D90=9μm. Mix the pulverized vicagrelor hydrochloride with microcrystalline cellulose, lactose, sodium carboxymethyl starch, and hydroxypropyl methylcellulose in a three-dimensional mixer at 35 rpm for 10 minutes, take it out and put it in a high-shear wet granulator, and stir at 500 rpm , cutting at 1000rpm and adding water to granulate, passing through a 16-mesh sieve for granulation, drying in a blast drying oven at 60°C, taking it out, passing through a 24-mesh sieve for granulation, adding magnesium stearate and blending. The drug-containing granules are filled into No. 3 capsules to obtain the vicagrel immediate-release capsules.
Embodiment 3
[0065] Embodiment 3 Vicarrel capsules
[0066]
[0067]
[0068] Put the pulverized vicagrel salt, pregelatinized starch, lactose, and sodium carboxymethyl starch in a fluidized bed, start the fluidized mixing for 10 minutes, and prepare 5% hydroxypropyl methylcellulose as a binder. The air inlet temperature is 80°C, and the binder is sprayed while maintaining the bed temperature at 40-50°C. After forming particles, maintain the bed temperature at 50-60°C and dry for 30 minutes. After discharging, add sodium stearyl fumarate and mix for 5 minutes. Capsules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com