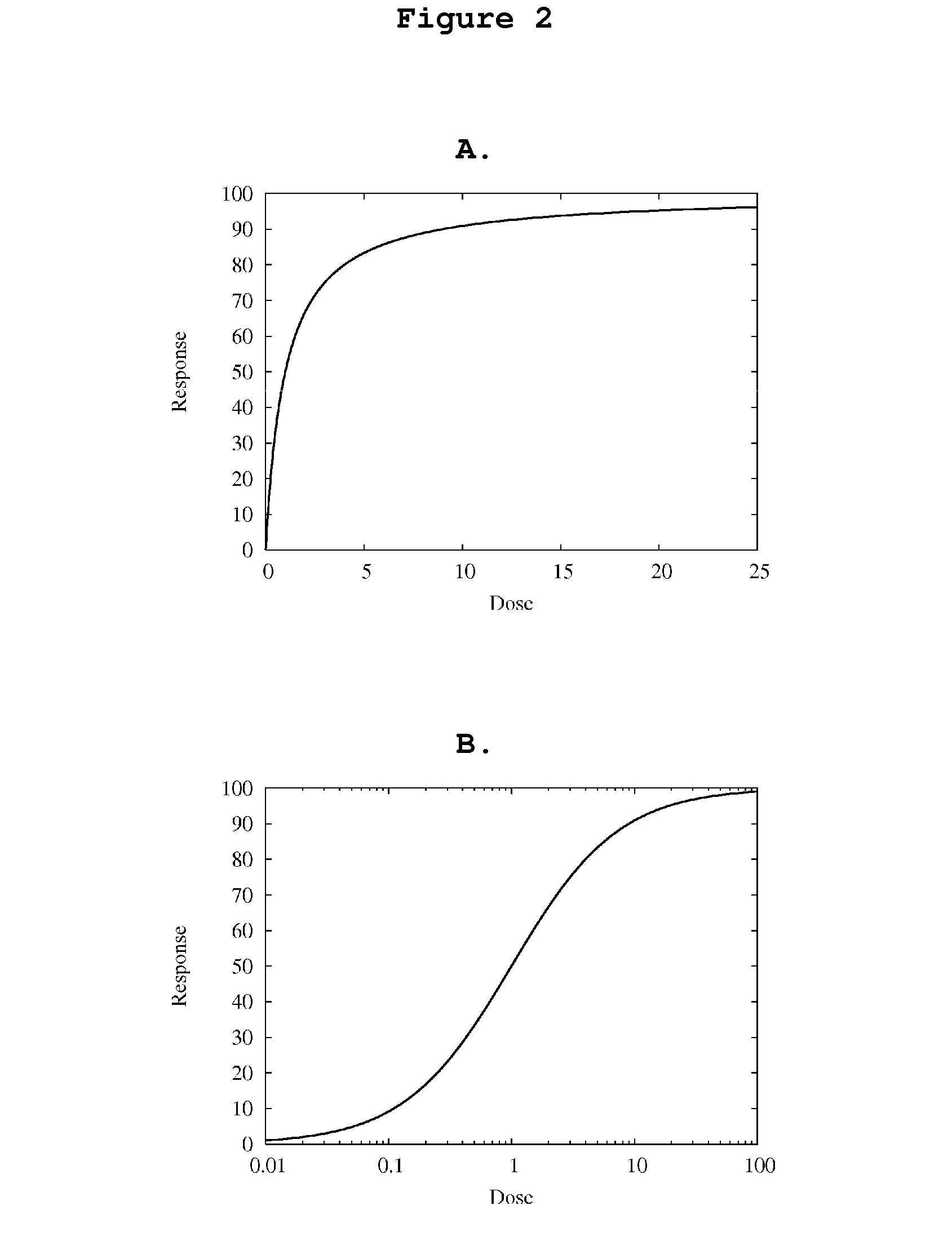
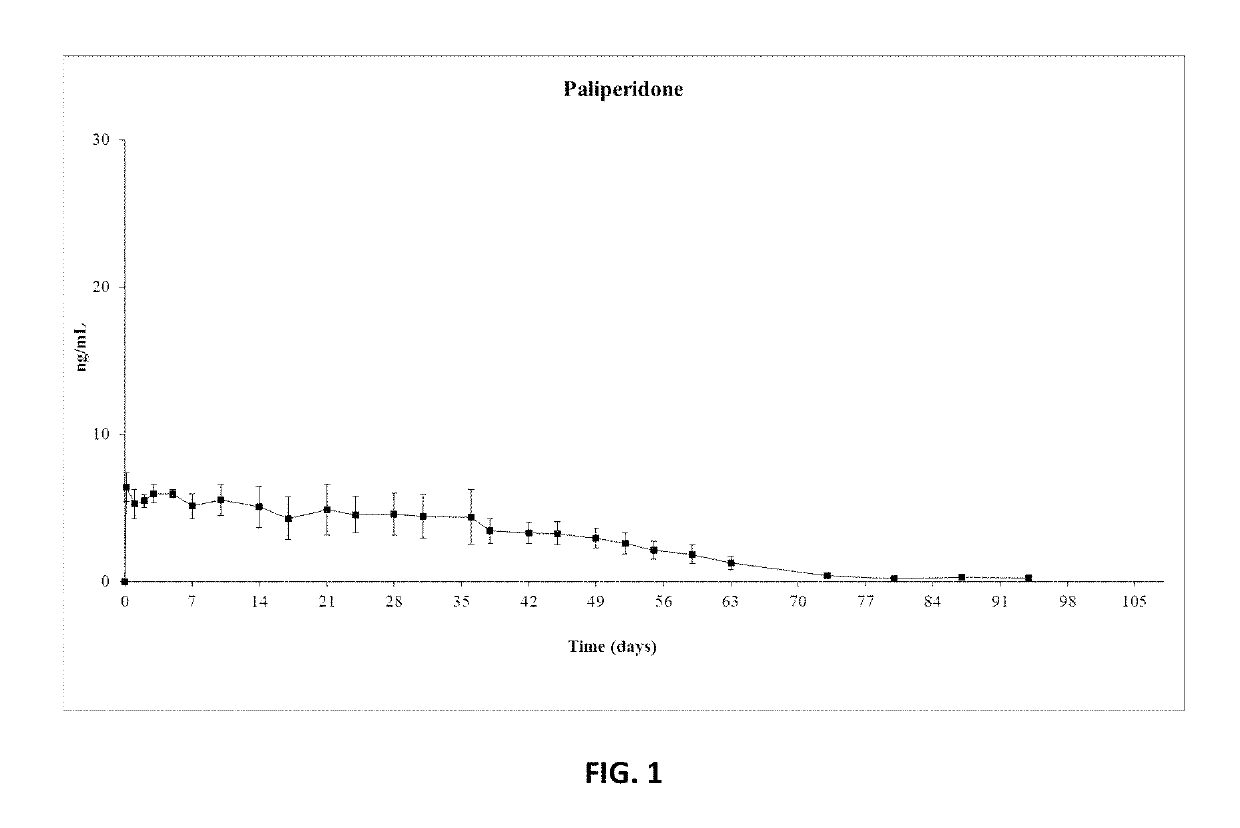
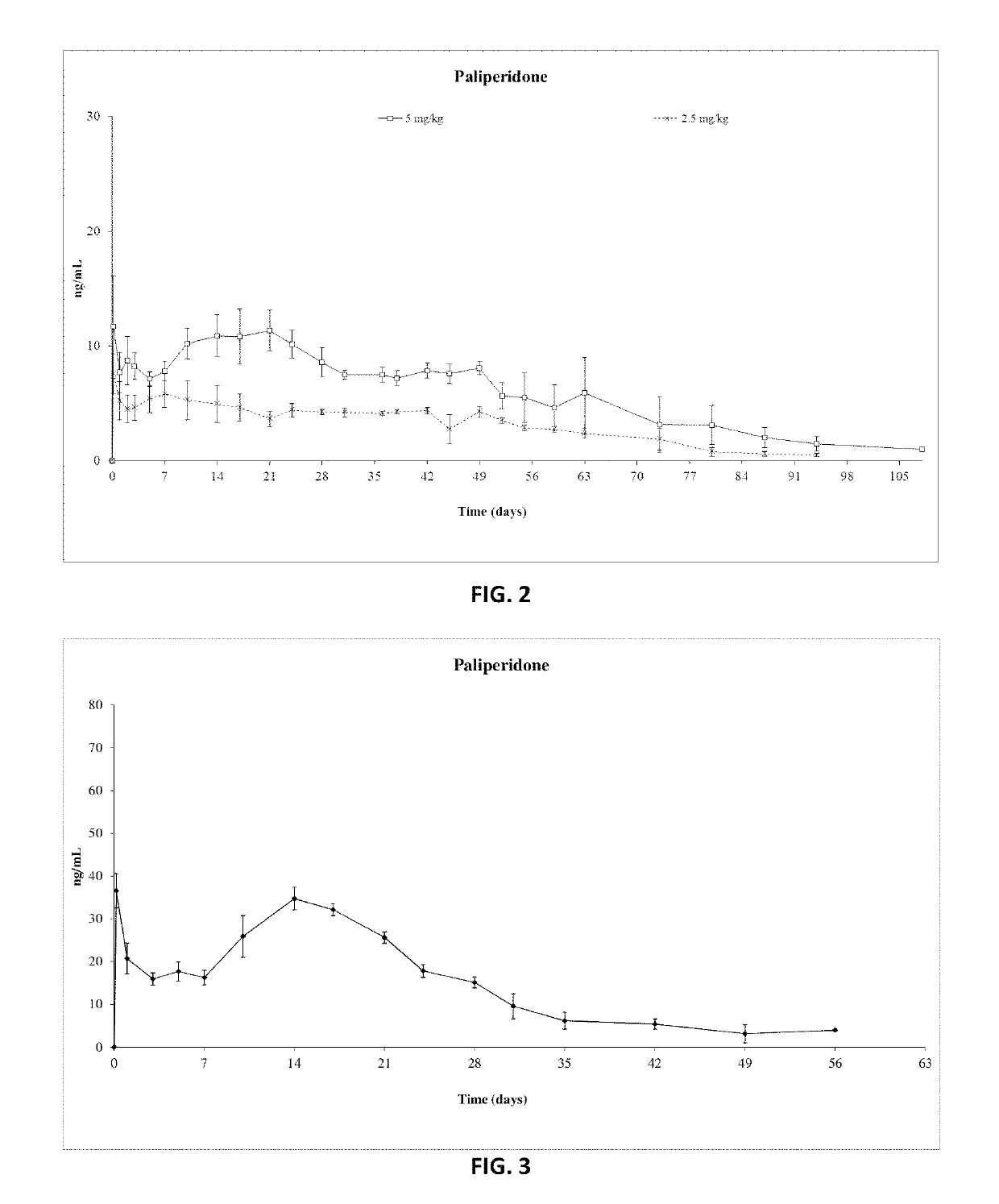
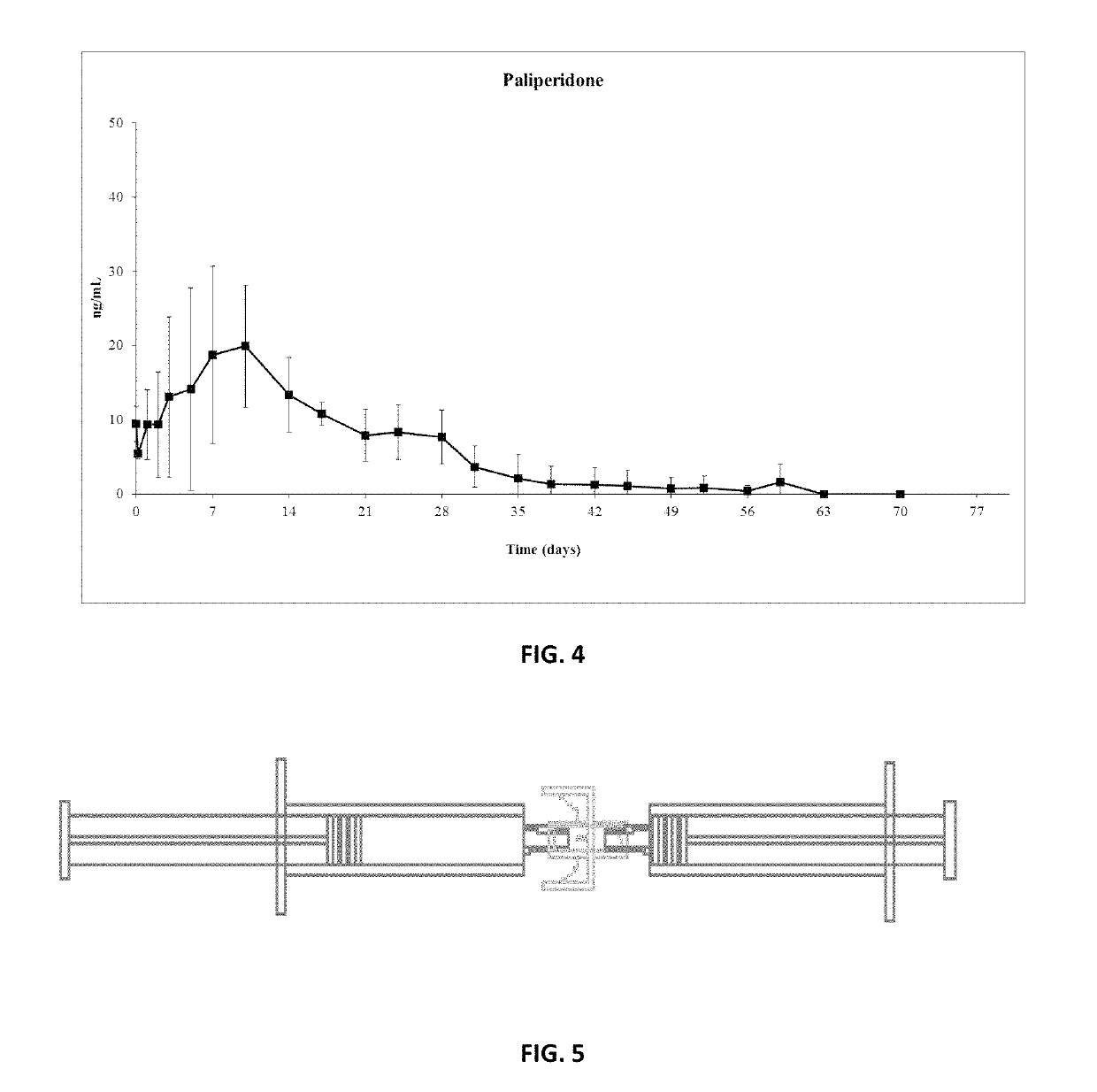
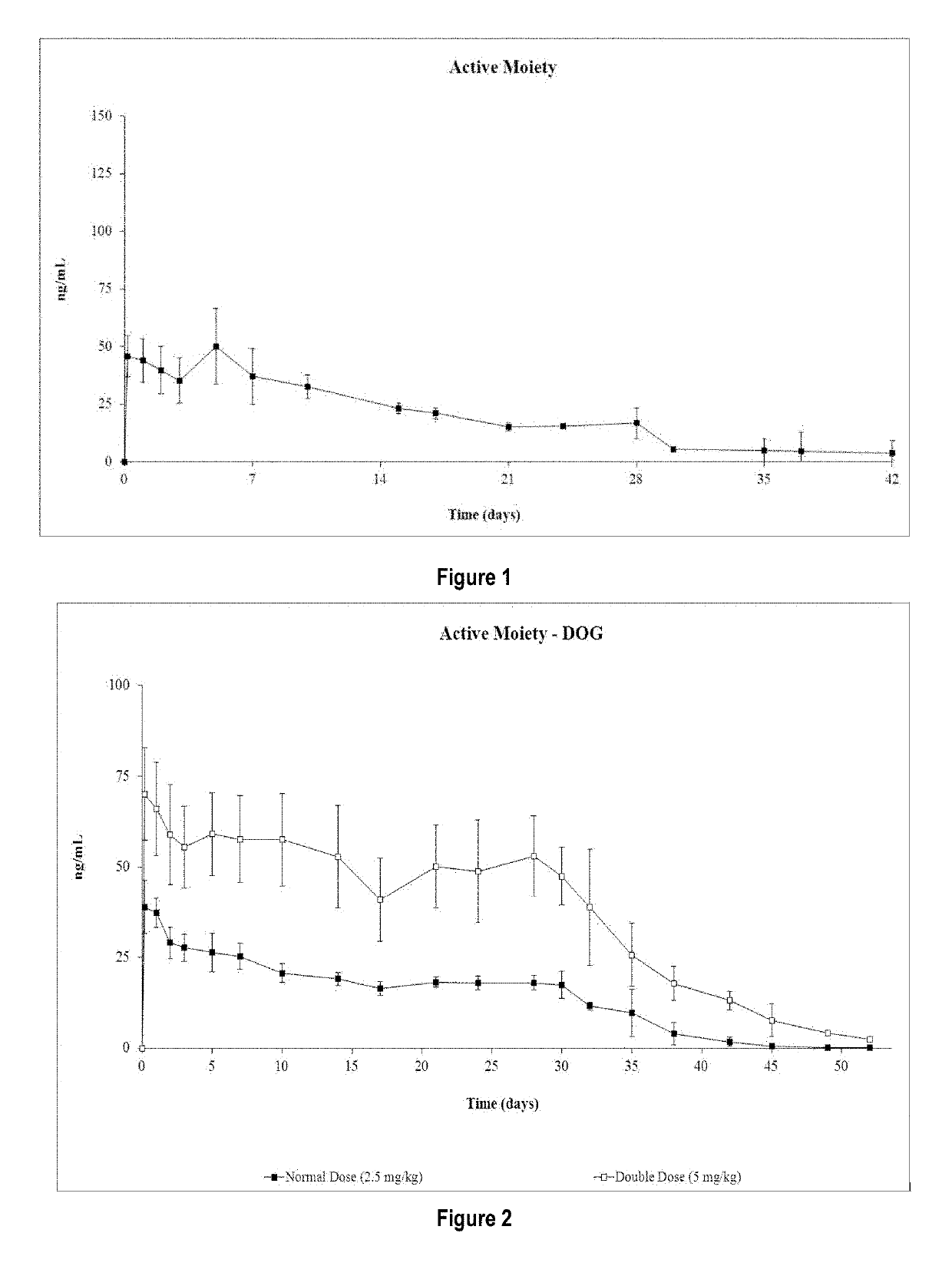
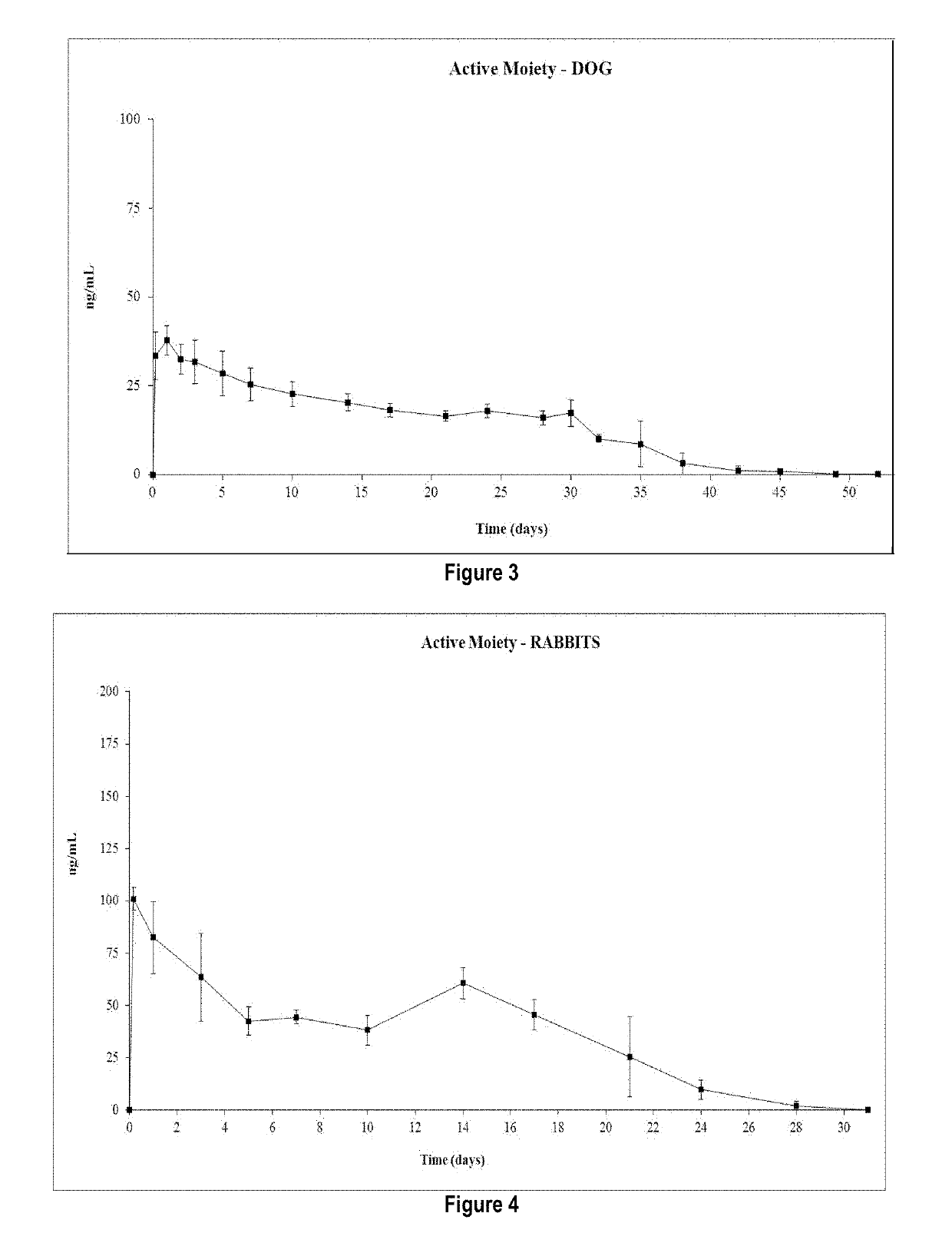
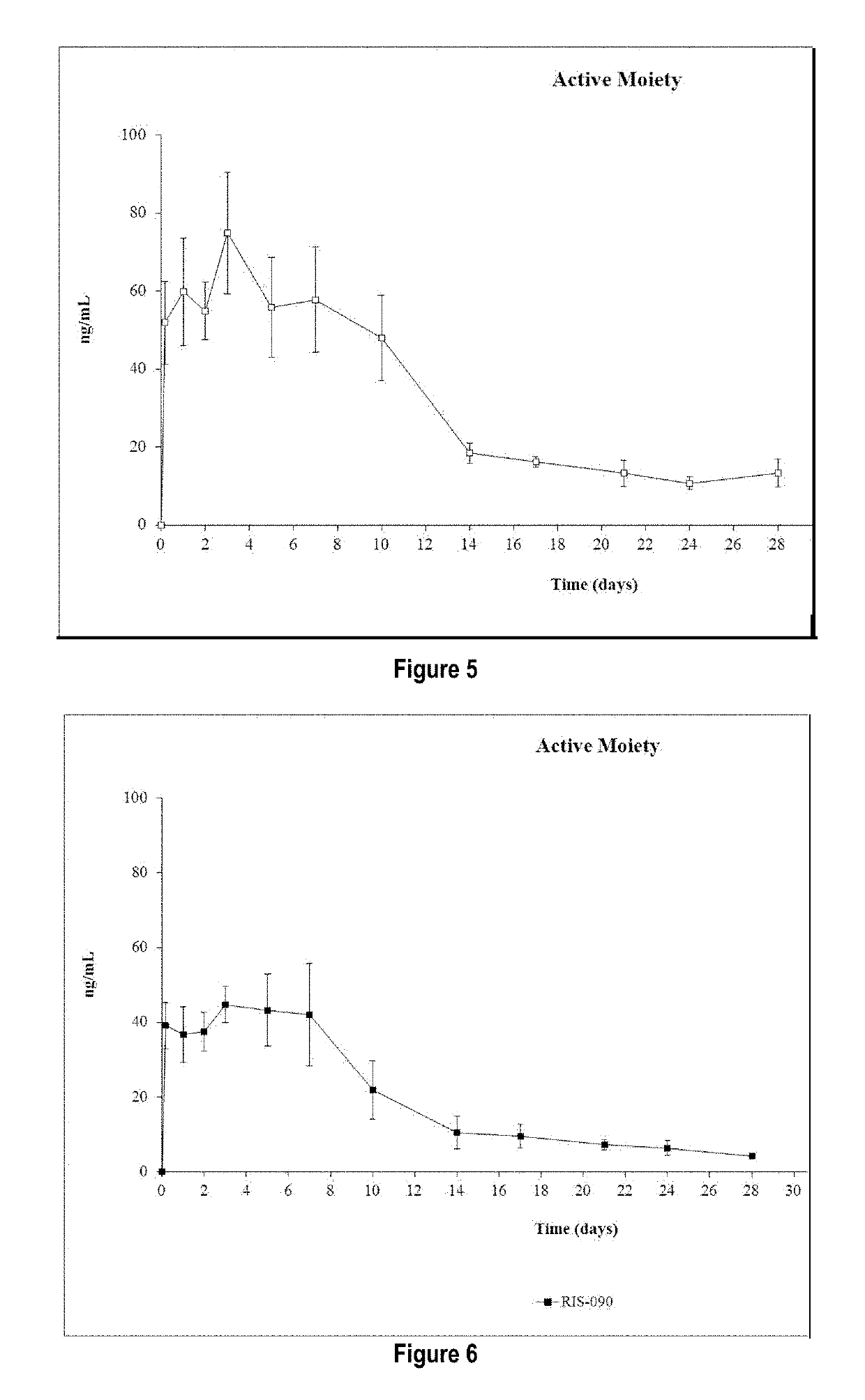
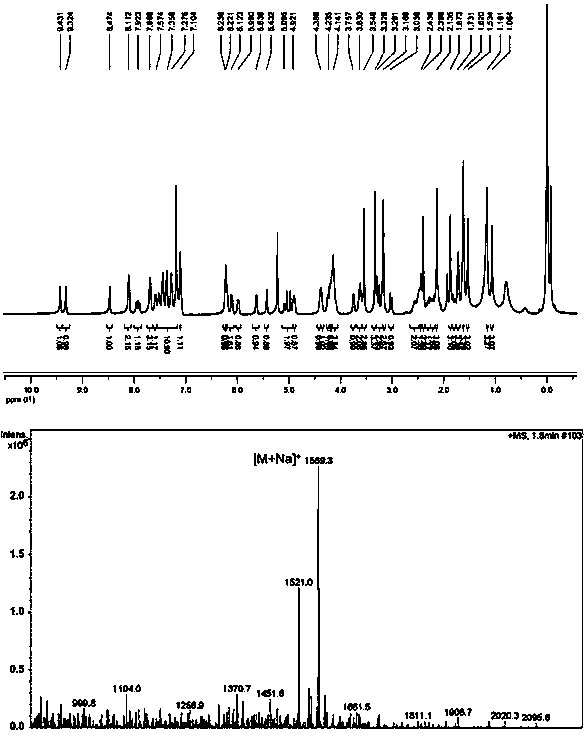
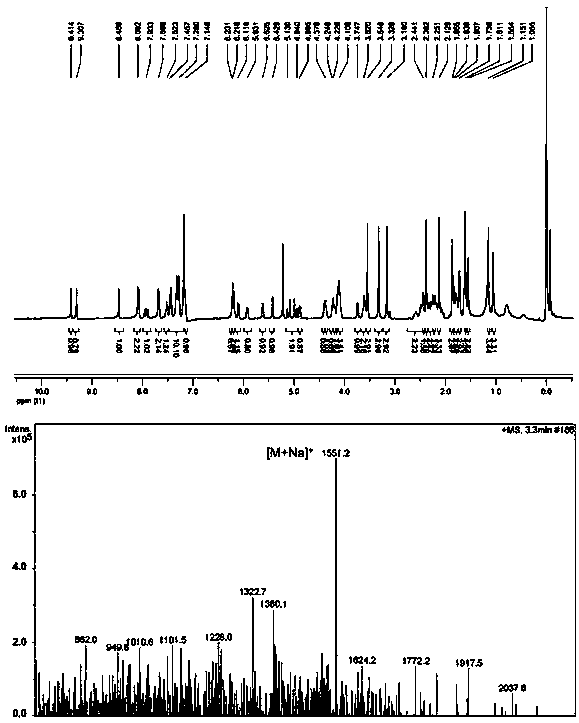
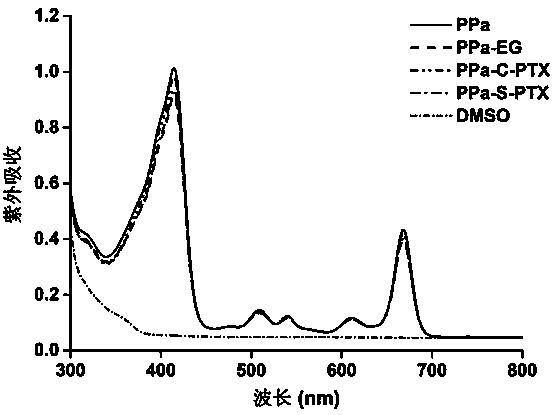
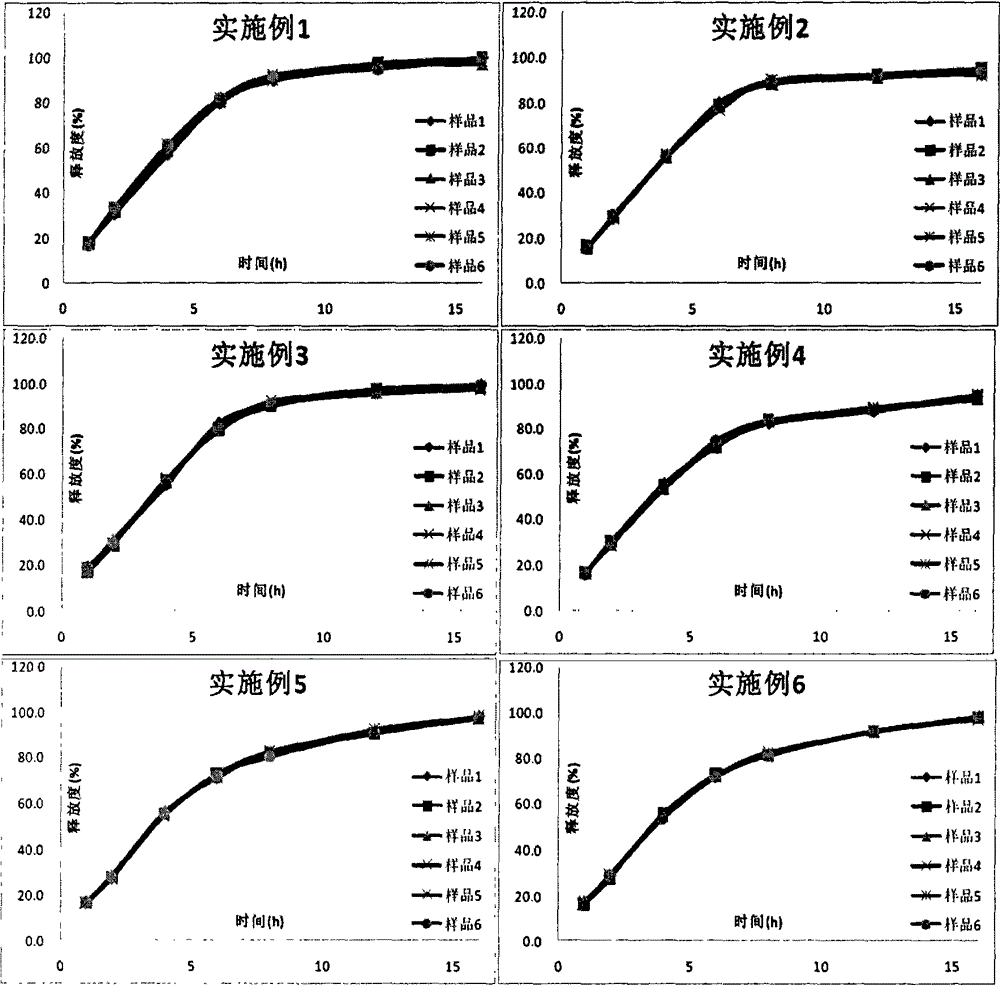
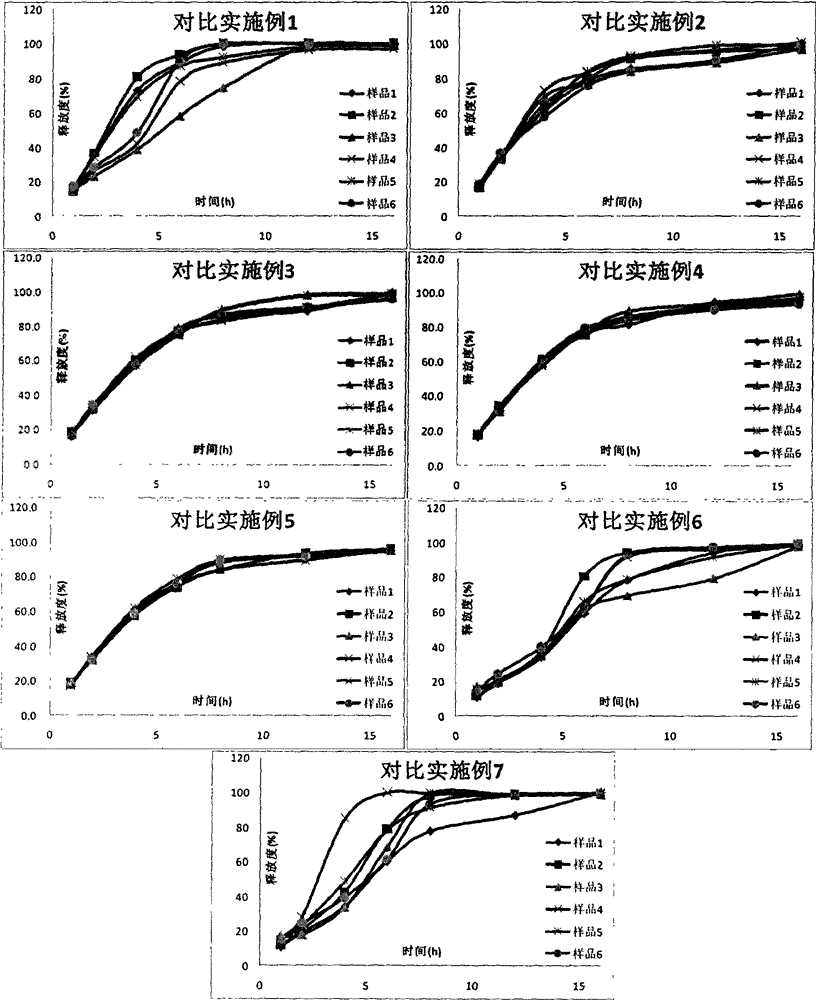
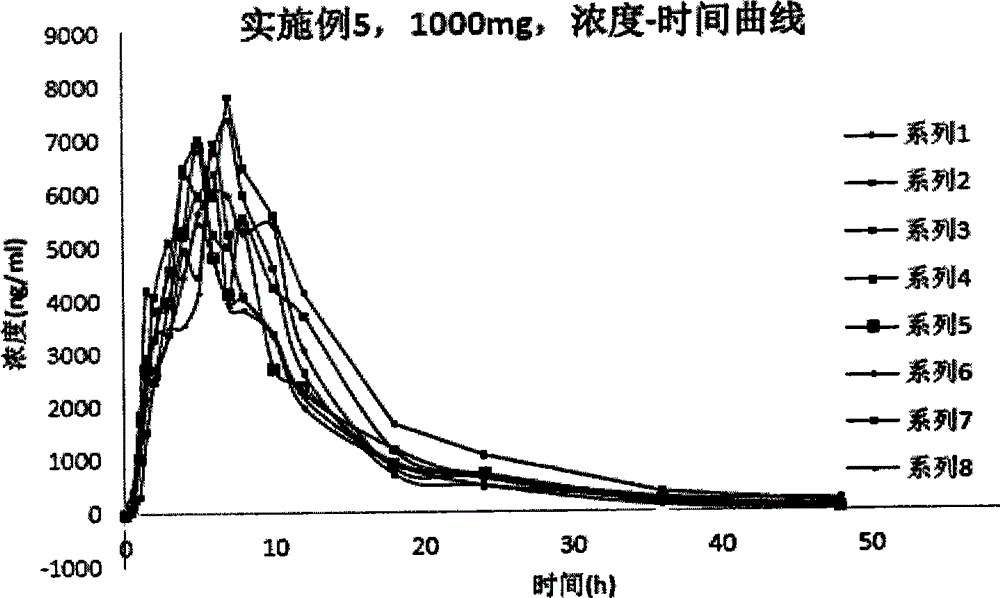
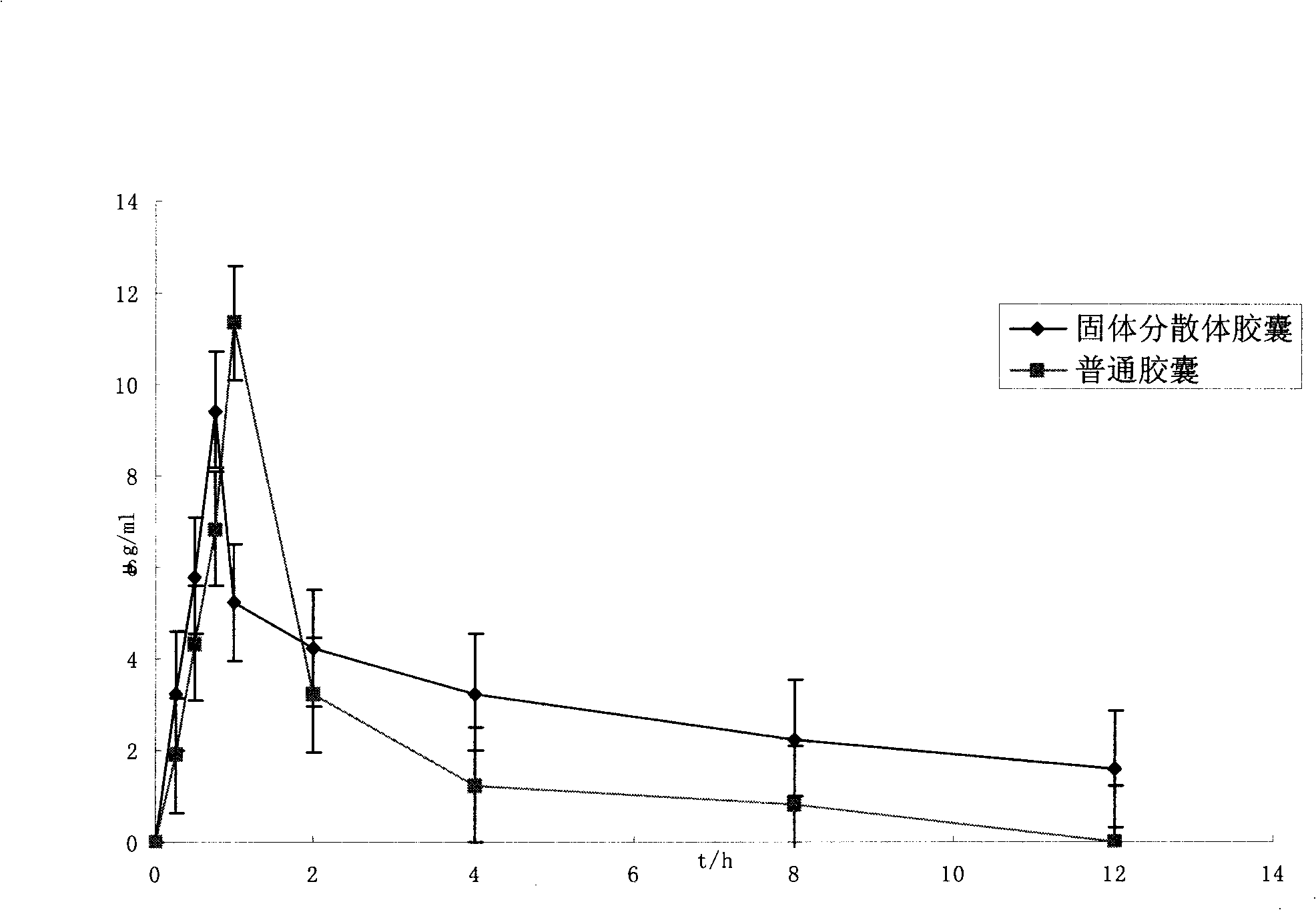
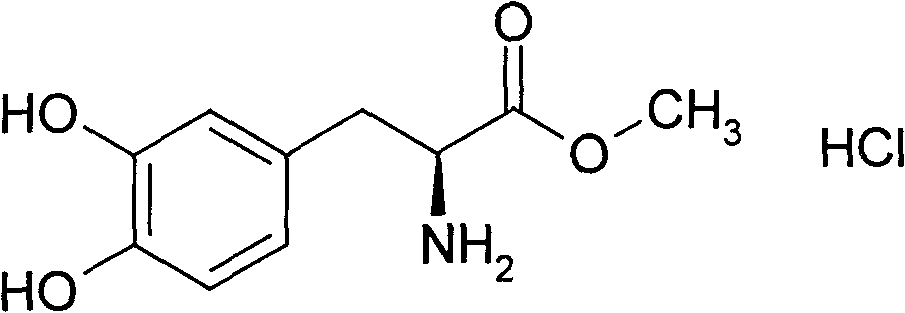
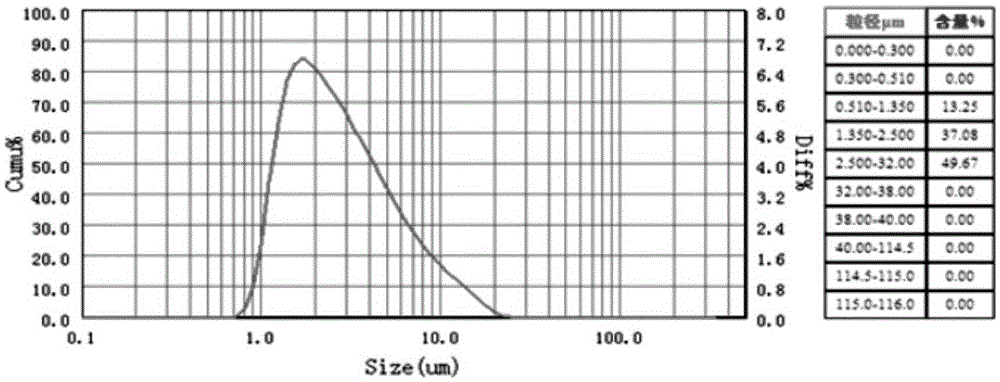
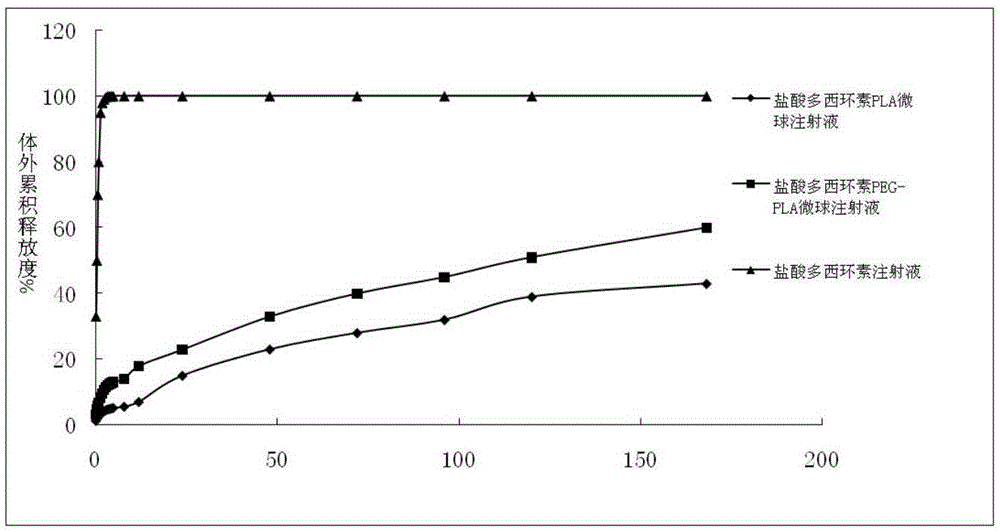
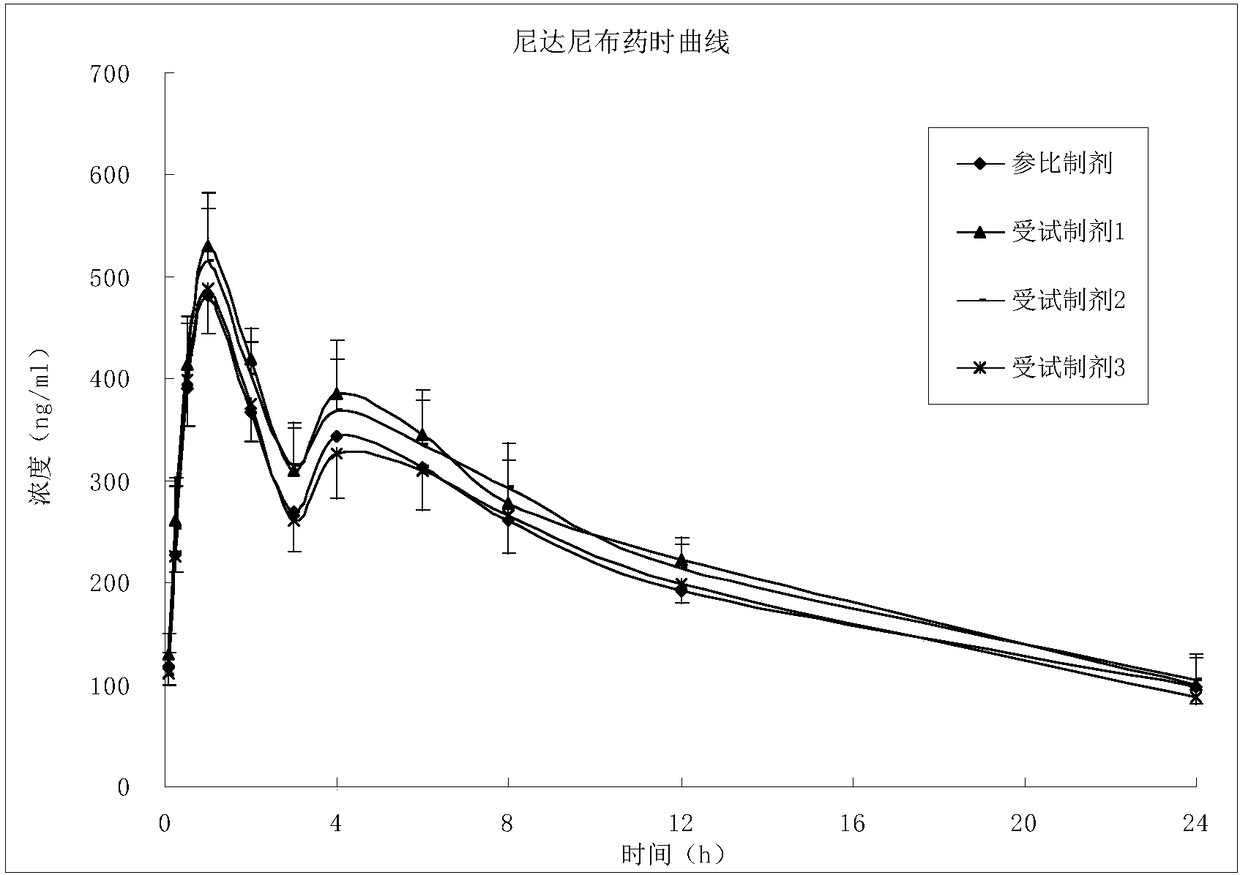
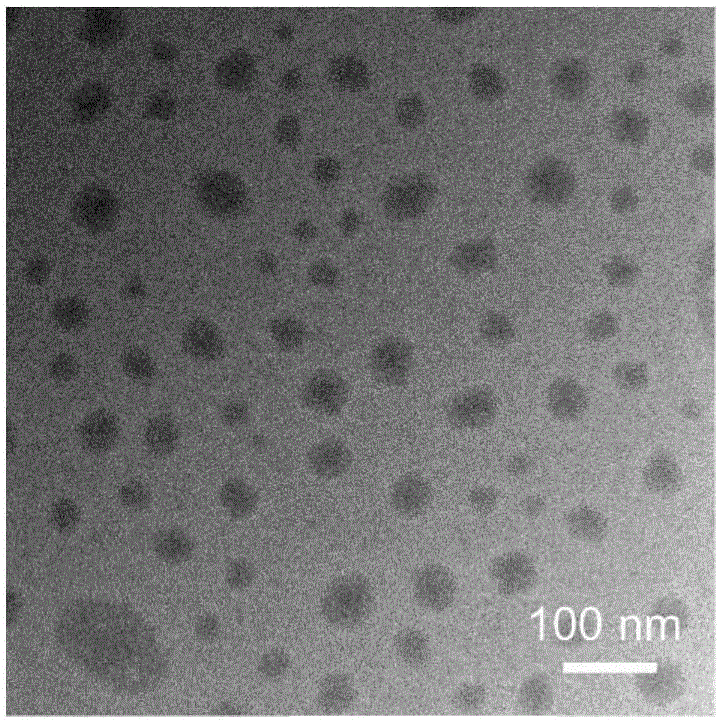
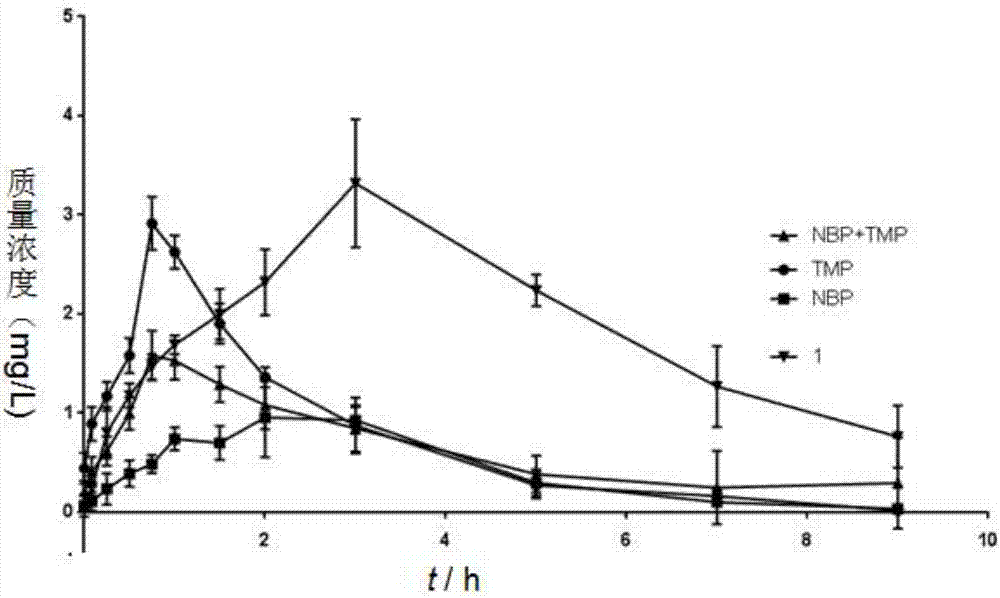
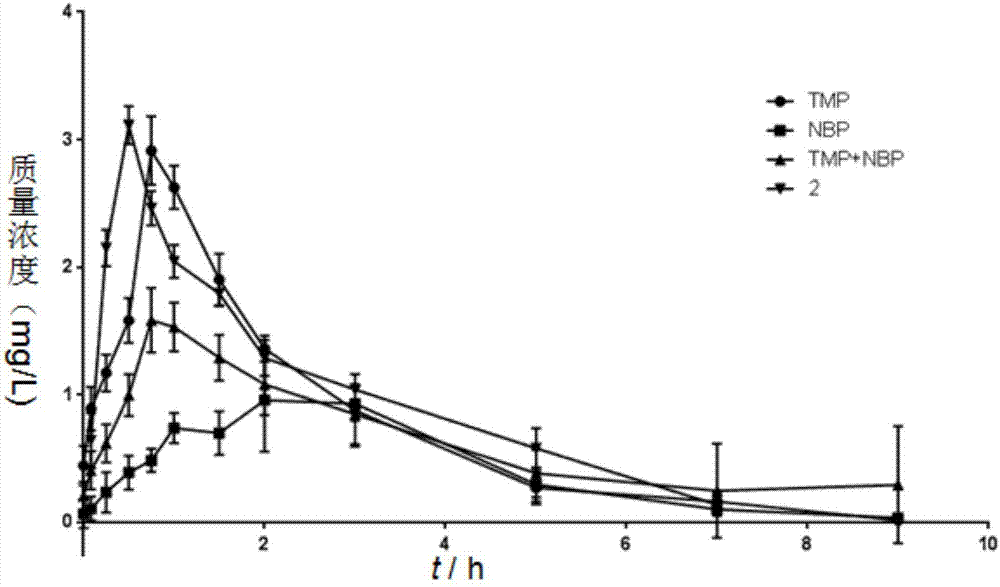
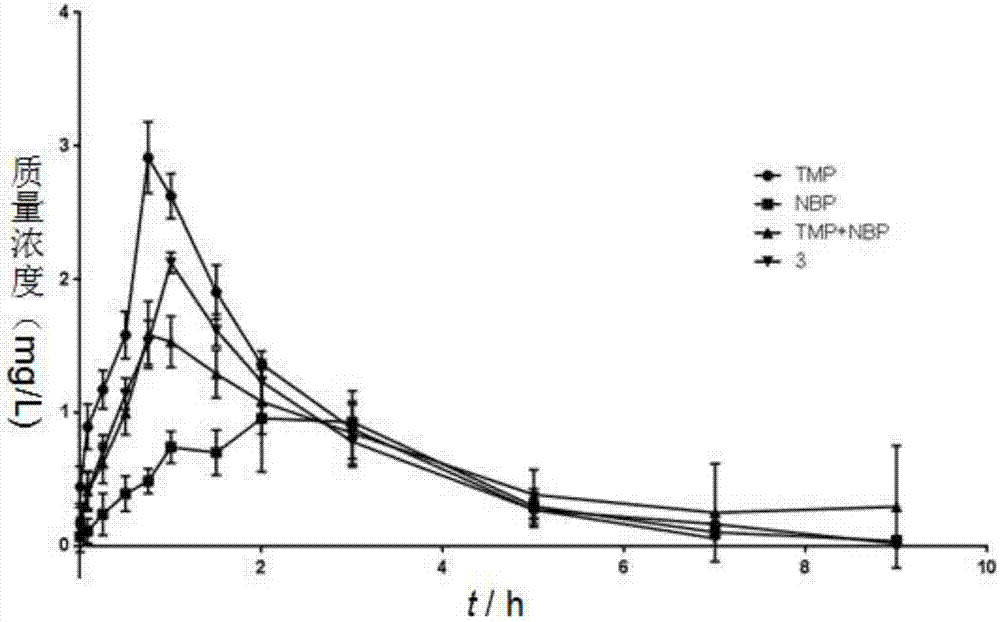
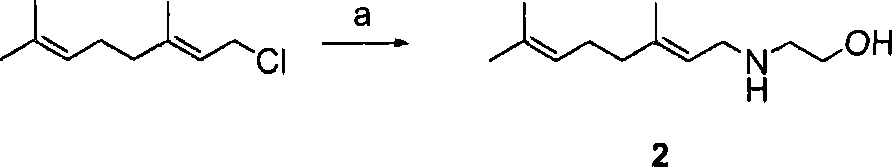
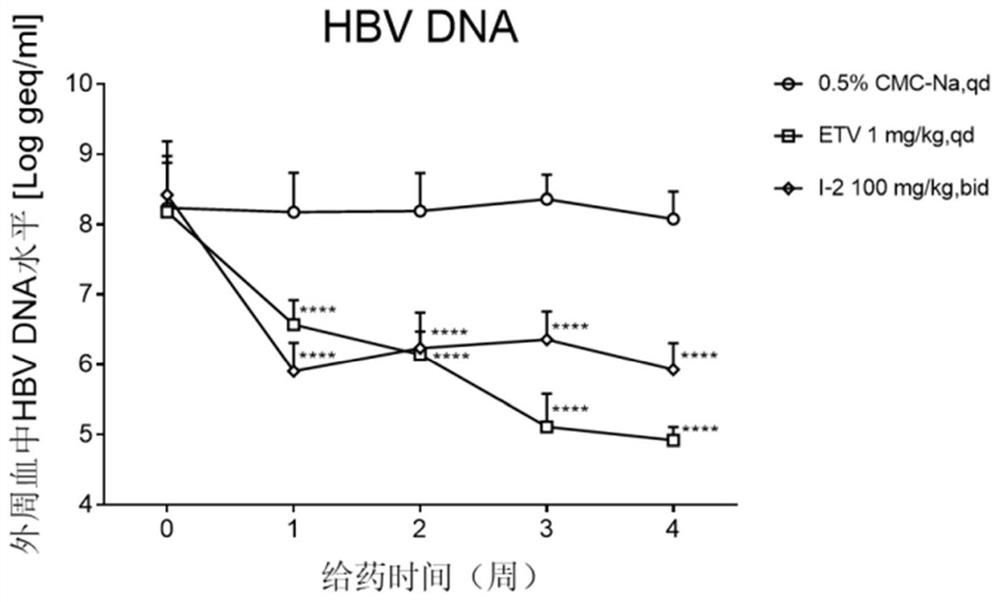
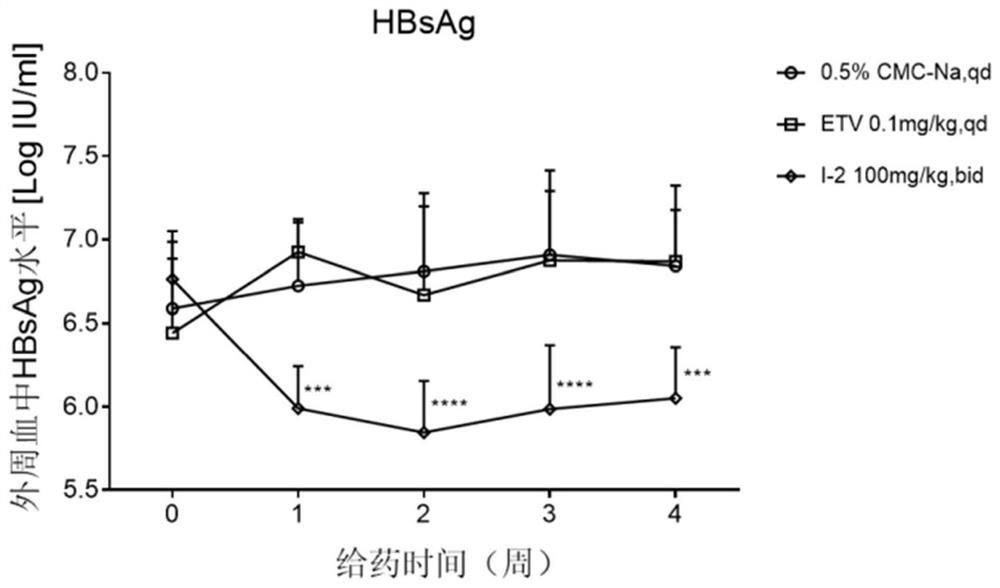
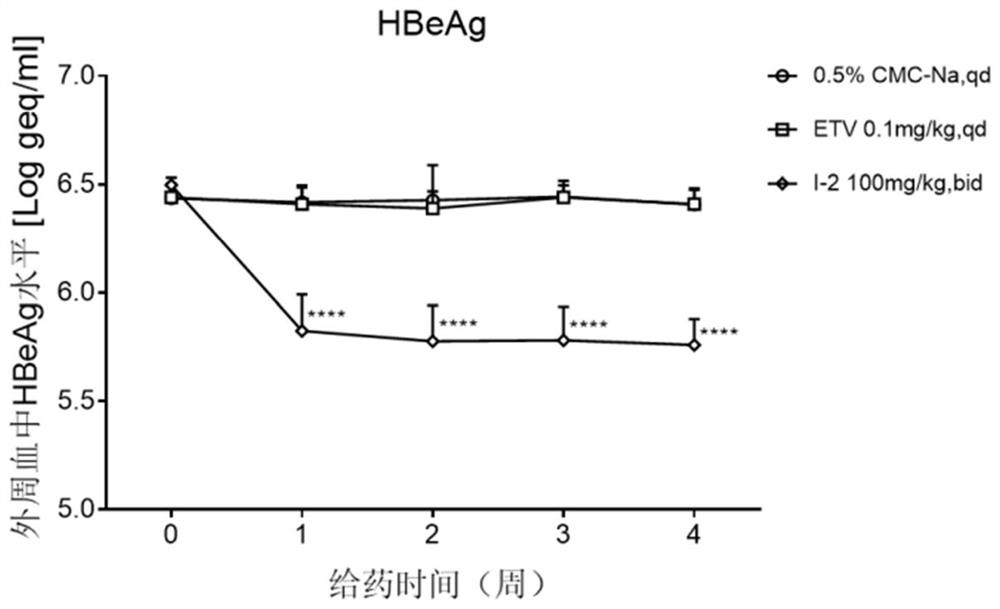
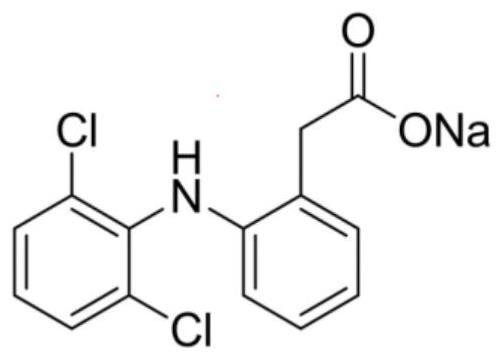
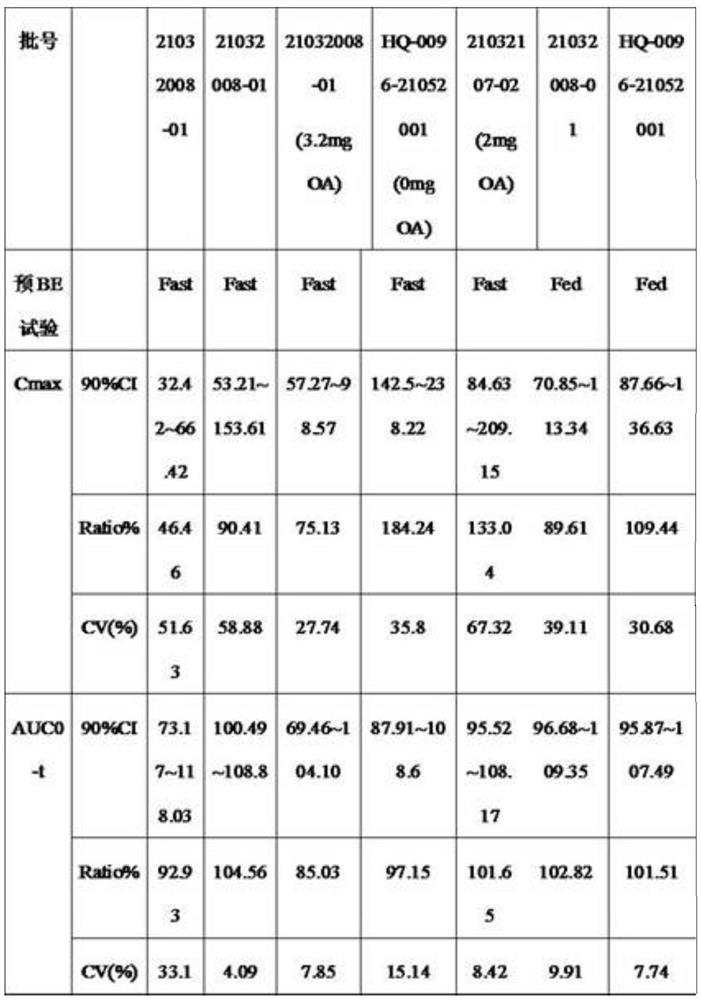
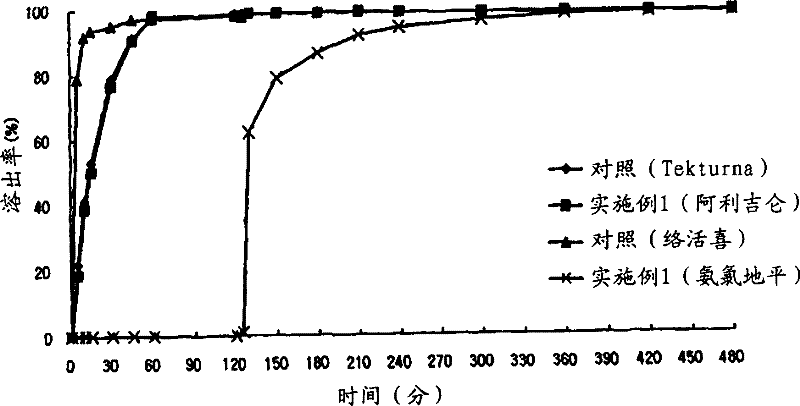
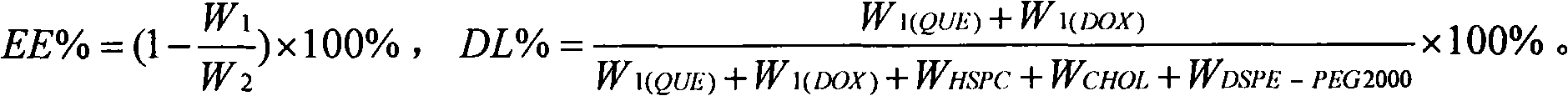Patents
Literature
85 results about "In vivo pharmacokinetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
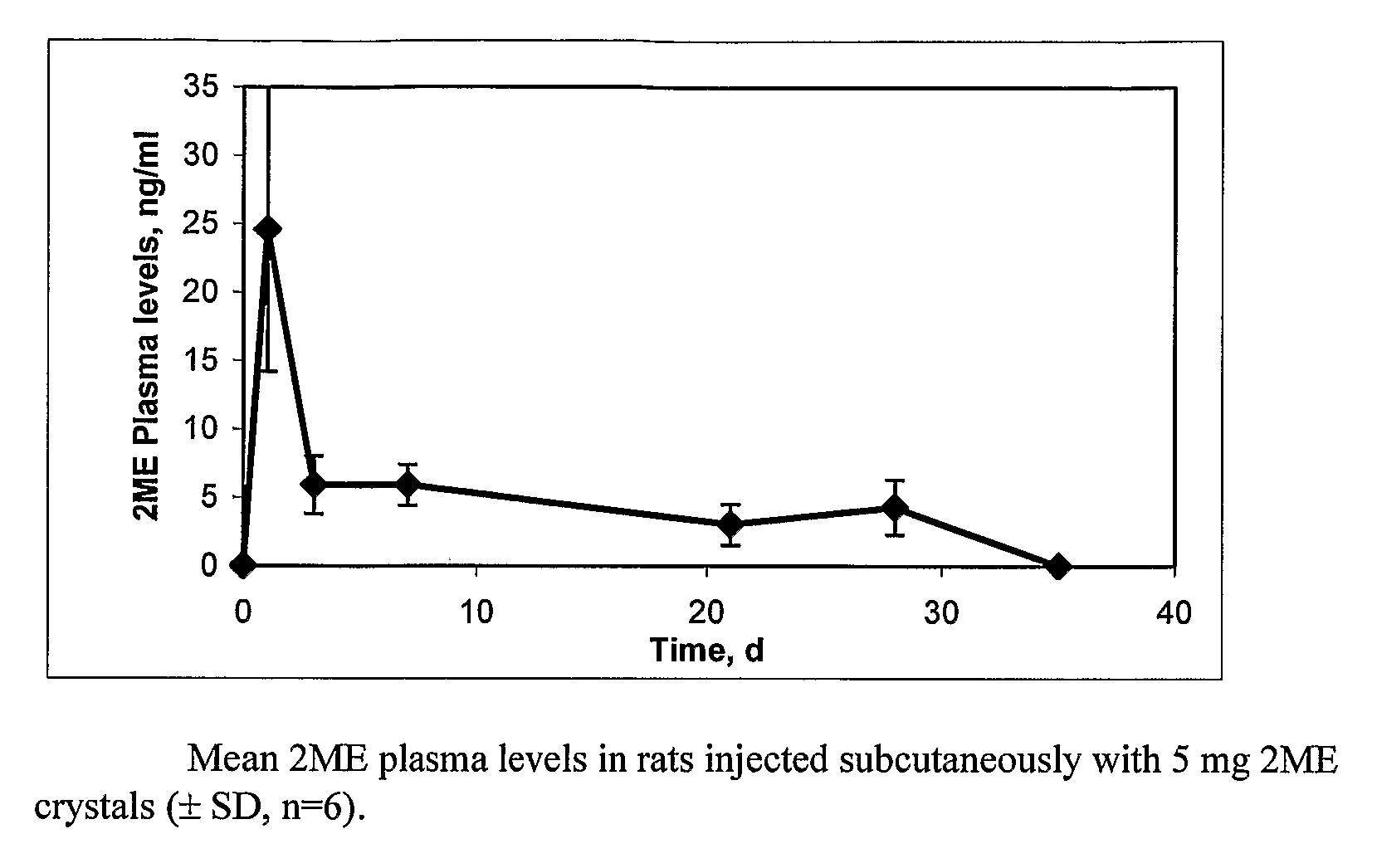
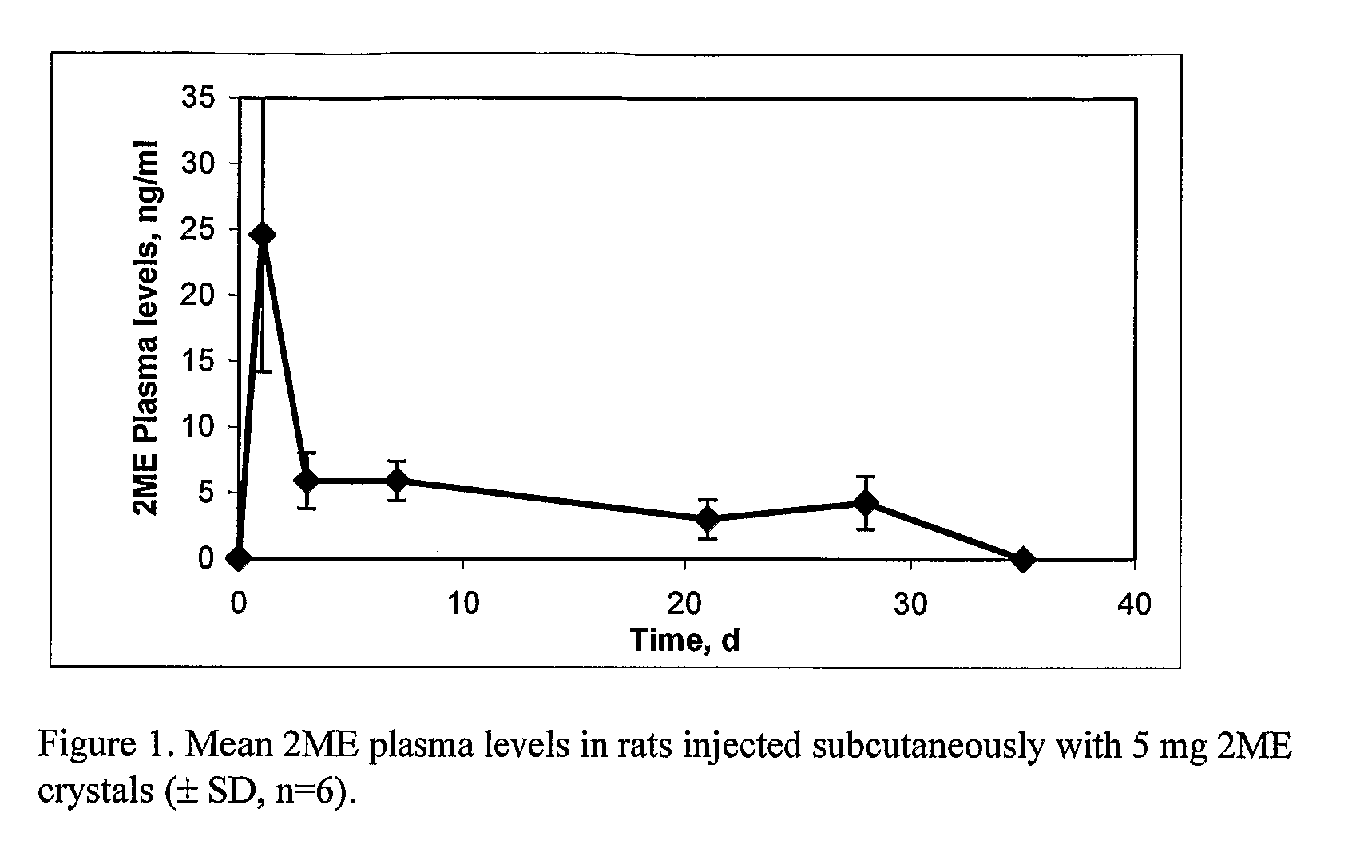
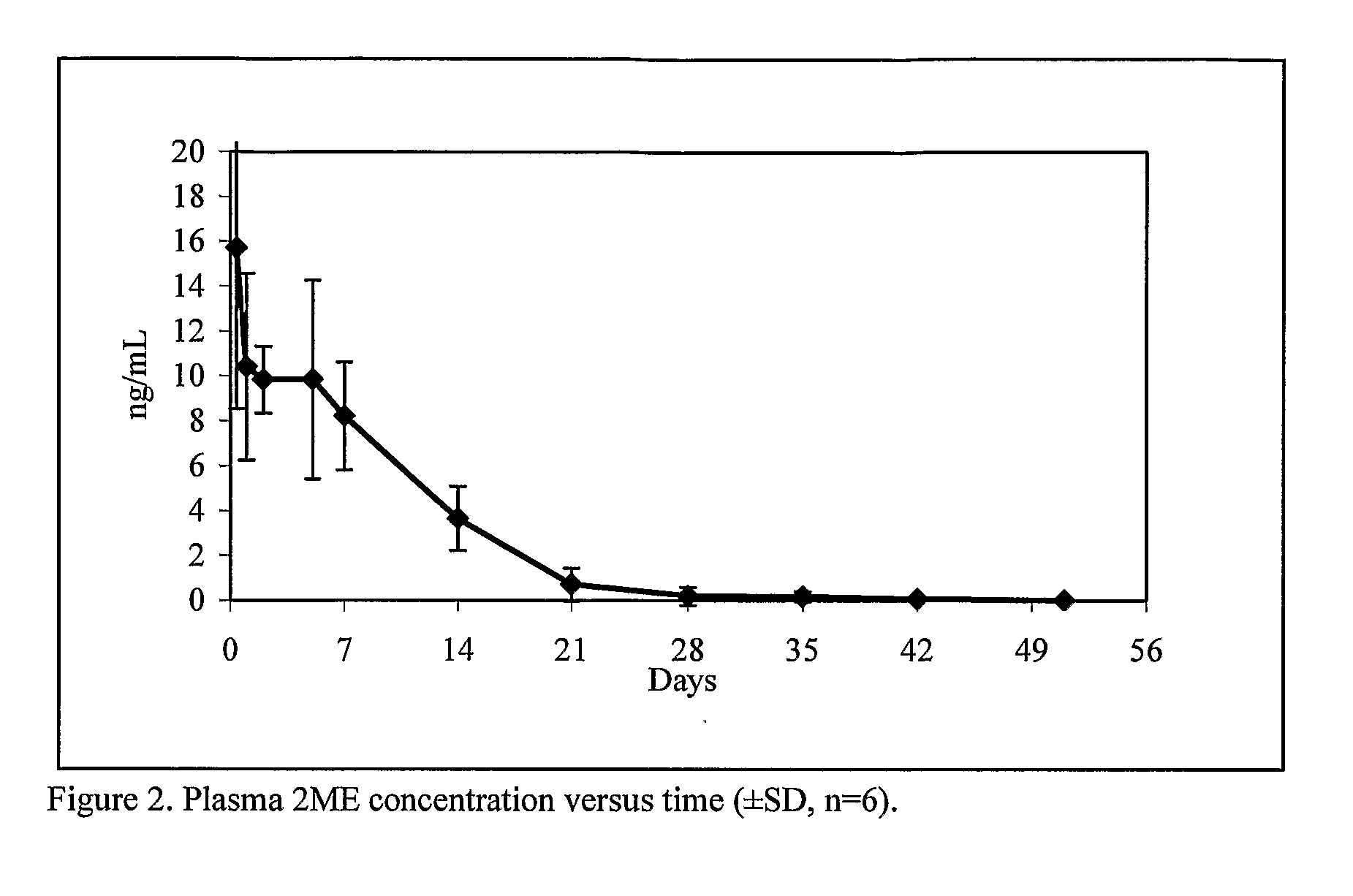
Long Acting Injectable Crystal Formulations of Estradiol Metabolites and Methods of Using Same
InactiveUS20080220069A1Simpler and less-expensive to manufactureEasy to managePowder deliveryOrganic active ingredientsDiseaseMetabolite
The present invention provides sustained release formulations of estradiol metabolites whereby the in vivo pharmacokinetics are manipulated by a method selected from the group consisting of chemical modification, crystal packing formation, particle size or a combination thereof. Such compositions are useful in the long-term treatment of a wide variety of diseases.
Owner:PR PHARMA
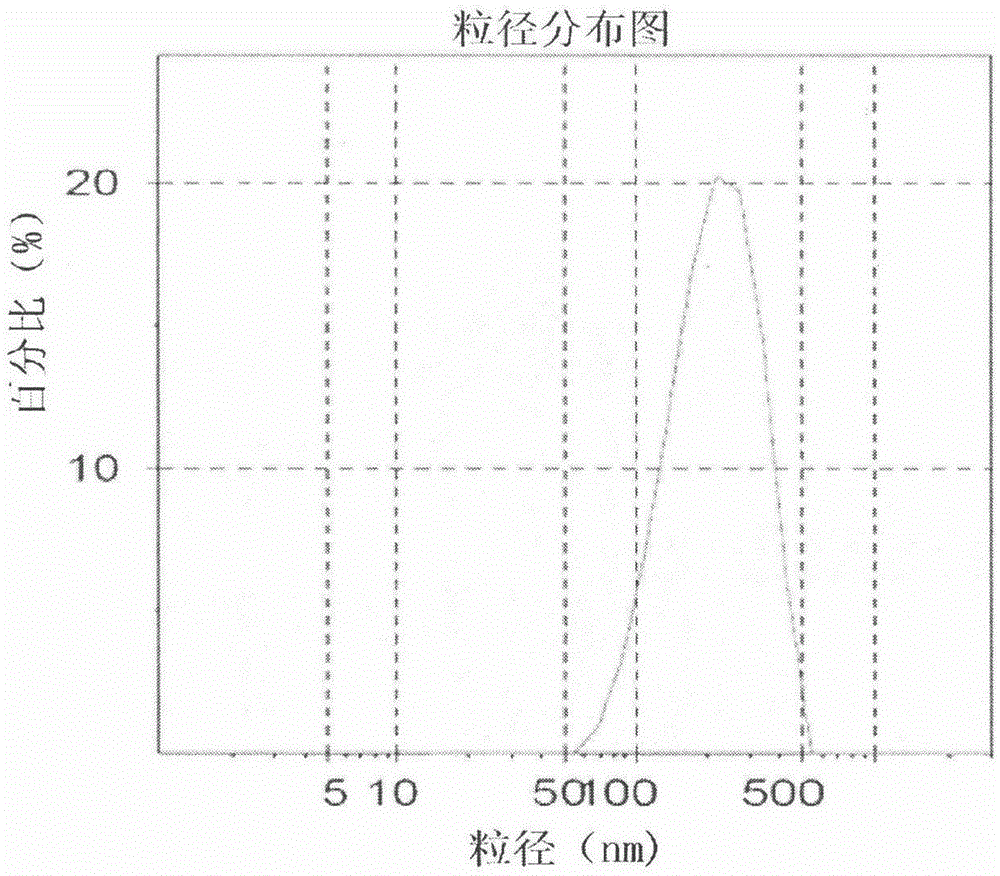
Celecoxib nanosuspension and preparation method thereof
InactiveCN105147607ASimple prescriptionSimple processOrganic active ingredientsAntipyreticSolubilityDissolution
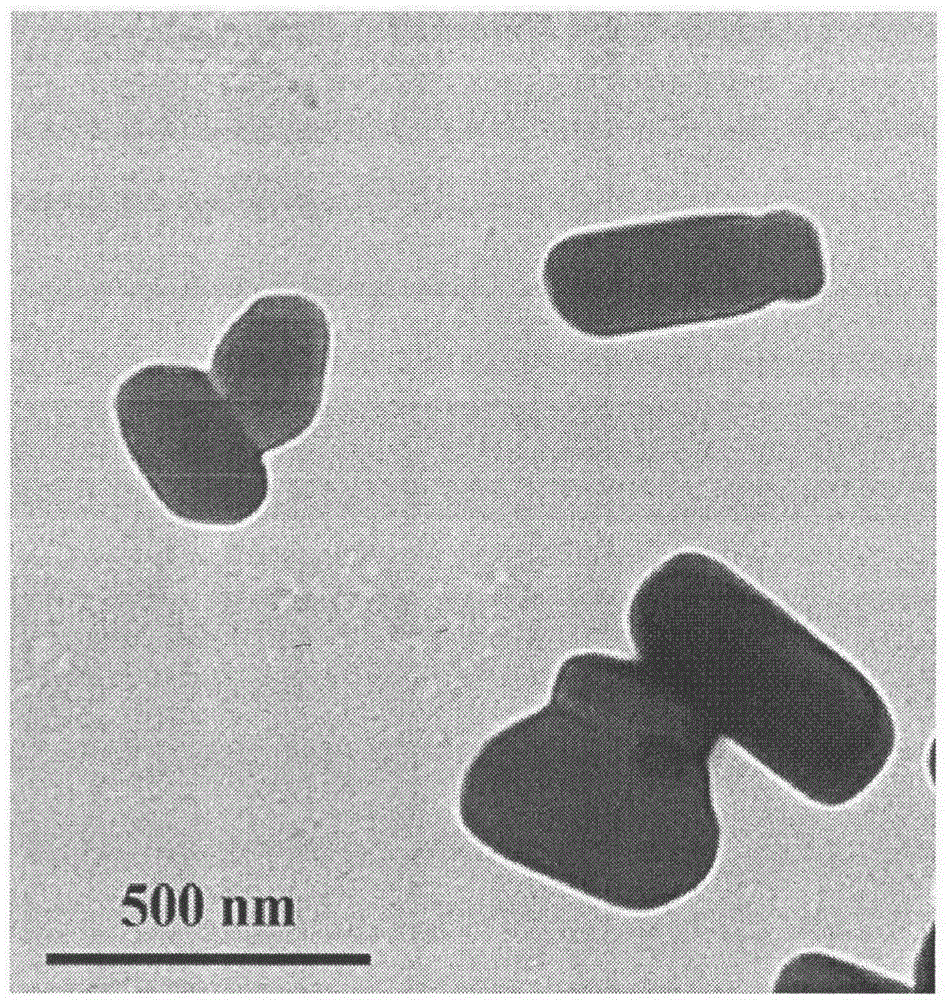
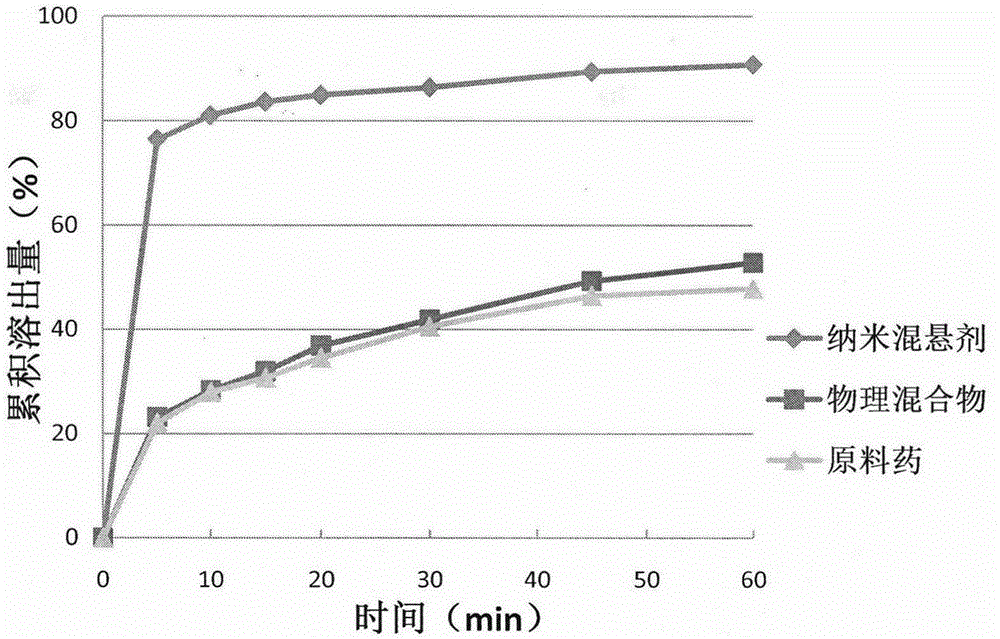
The invention belongs to the field of pharmaceutical preparations and provides a Celecoxib nanosuspension and a preparation method thereof. Celecoxib is a novel non-steroidal anti-inflammatory drug, inhibits cyclooxygenase -2(COX-2) is through specificity, has anti-inflammatory and pain-easing effects and is clinically used for treating osteoarthritis and rheumatoid arthritis. However, the Celecoxib has very low solubility and in-vivo bioavailability. In order to increase the dissolution and the bioavailability of the drug, a high-speed shearing combined high-pressure homogenizing method is adopted to prepare the Celecoxib nanosuspension, the particle size and the polydispersion index PI are used as indicators for formulation technology optimization, a laser particle analyzer and a transmission electron microscope are adopted to study the particle size and form of the drug, the dissolution of the drug is evaluated through in-vitro dissolution experiments, and rat in-vivo pharmacokinetic study is conducted. Measurement results prove that the drug particle size of the nanosuspension is 50-500 nm, and the dissolution and the in-vivo bioavailability of the drug are increased obviously.
Owner:CHINA PHARM UNIV
Pharmaceutical Platform Technology for the Development of Natural Products
The present invention provides a set of in vitro and in silico methodologies for predicting in vivo pharmacokinetics and pharmacodynamics of multiple components; the methodologies comprise mathematical models for solving multiple unknowns which are linearly independent and / or interacting with each other. The present invention can be applied to develop phytomedicines which contain multiple active ingredients without prior identification, isolation and purification of these components.
Owner:SINOVEDA CANADA INC
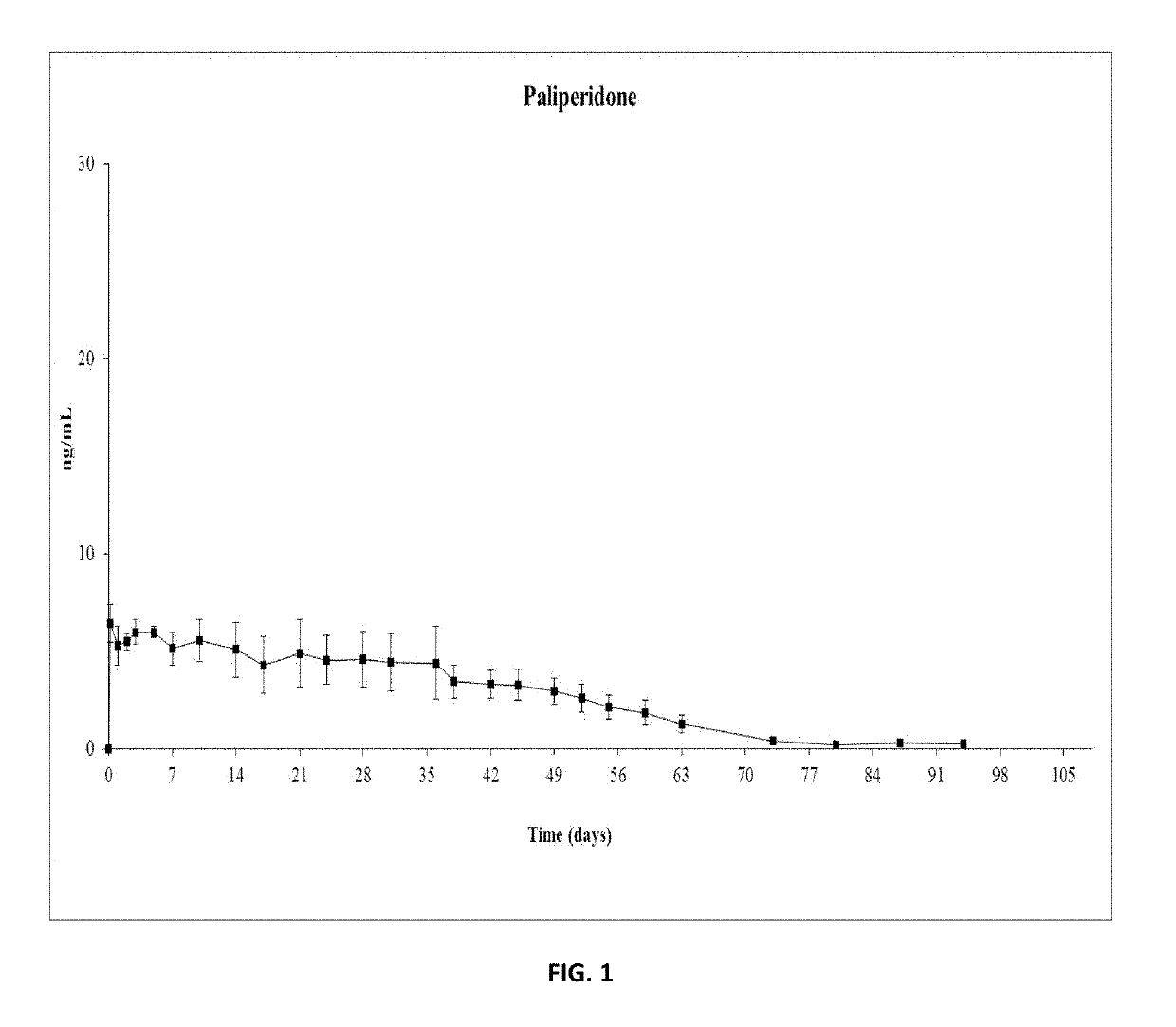
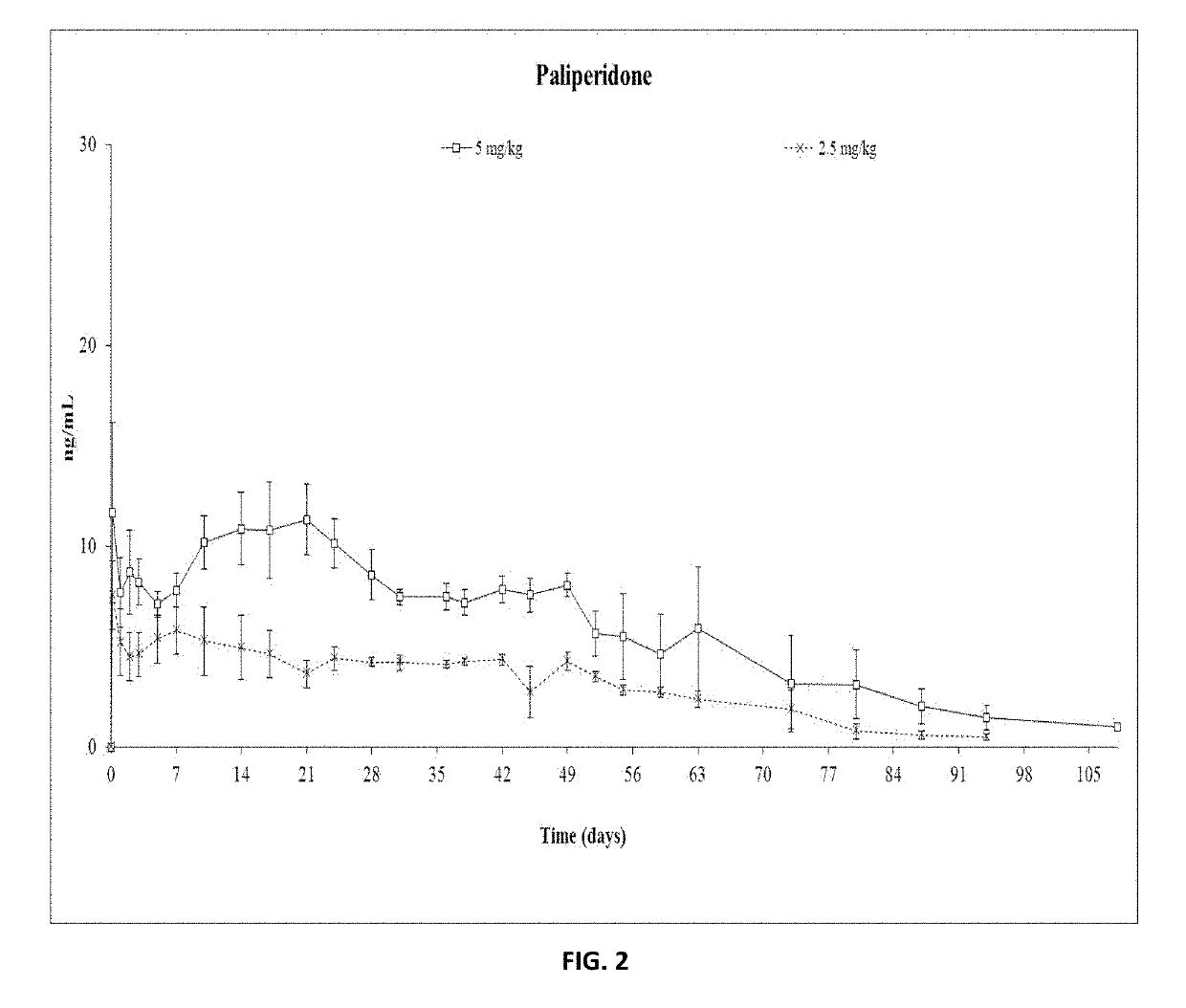
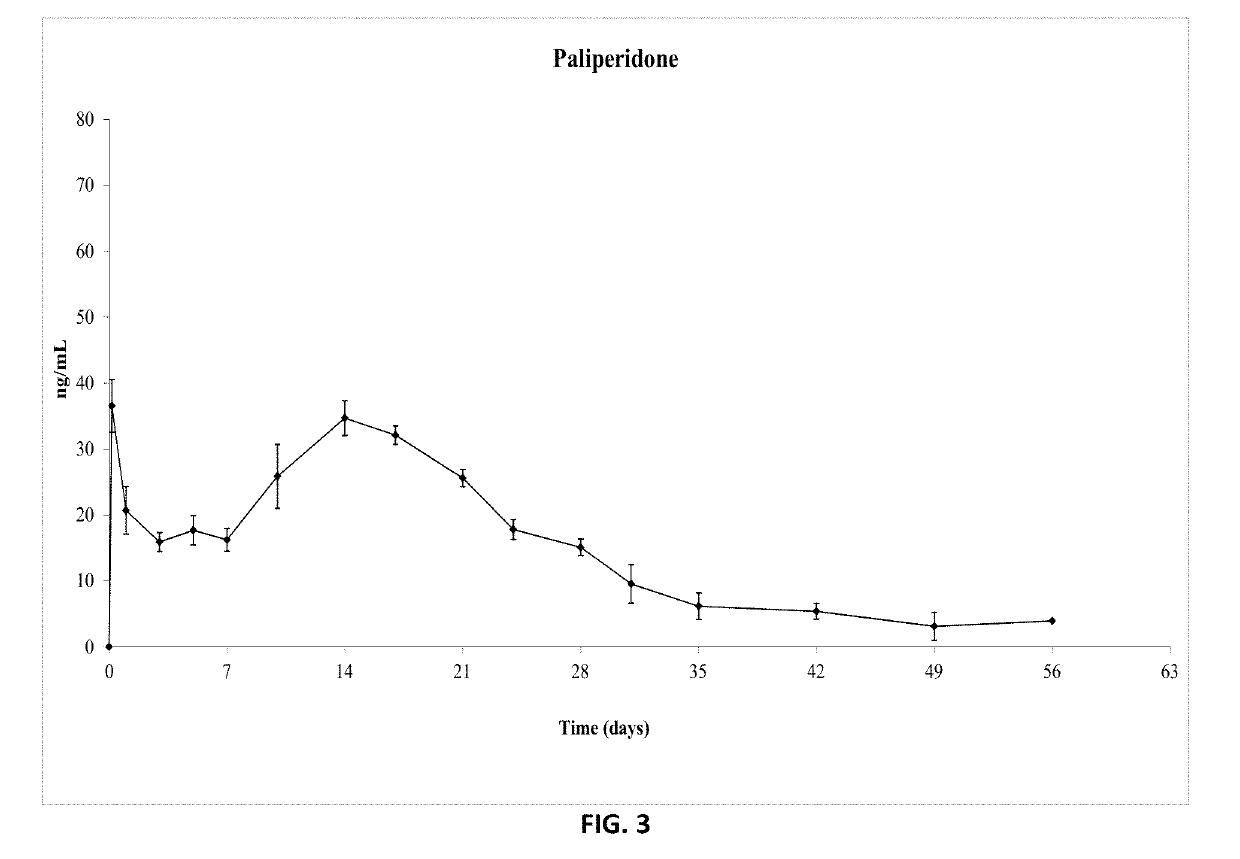
Paliperidone implant formulation
ActiveUS10350159B2Organic active ingredientsPharmaceutical delivery mechanismGlycolic acidPharmaceutical drug
An injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is paliperidone and / or its pharmaceutical acceptable salts in any combination thereof, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and DMSO as solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 8 weeks and wherein the composition has a pharmacokinetic profile in vivo suitable for the formulation to be administered each 8 weeks or even longer periods.
Owner:LAB FARM ROVI SA
Risperidone or paliperidone implant formulation
The present invention is directed to an injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is risperidone and / or paliperidone or any pharmaceutically acceptable salt thereof in any combination, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and a DMSO solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 4 weeks and wherein the composition has a pharmacokinetic profile in vivo that makes it suitable to be administered each 4 weeks or even longer periods.
Owner:LAB FARM ROVI SA
Anti-tumor multi-medicine resistant targeted liposome
InactiveCN102357074AGood treatment effectEfficient killingOrganic active ingredientsPharmaceutical non-active ingredientsSide effectCytotoxicity
The invention relates to an anti-tumor multi-medicine resistant targeted liposome and a preparation method thereof, in particular to an anti-tumor multi-medicine resistant long circulating targeted liposome. The invention is characterized in that: quercitin and an anti-tumor medicine are jointly coated in the liposome, and simultaneously target tumor tissues to reverse the multi-medicine resistance of tumors, cytotoxicity of anti-tumor medicines on medicine resistant tumors is enhanced, the change of pharmacokinetic properties of the anti-tumor medicines in vivo and the enhancement of toxic and side effects of the anti-tumor medicines in vivo, which are caused by the difference and interaction of the pharmacokinetic properties of different medicines in vivo, are avoided, and the treatmenteffect on medicine resistant tumors is improved.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Photosensitizer-chemotherapeutic drug 'photosensitizer-chemotherapeutic integration' micromolecule prodrug and establishment of self-assembled nanoparticle thereof
ActiveCN108478794AIncreased sensitivityAvoid ingestionOrganic active ingredientsEnergy modified materialsWilms' tumorTumor cells
The invention belongs to the technical field of medicines, relates to a photosensitizer-chemotherapeutic drug 'photosensitizer-chemotherapeutic integration' micromolecule prodrug, and aims to connecta chemotherapeutic drug with a photosensitizer through ester bonds or mono-thioether bonds to achieve efficient co-carrying and synchronous delivery of the chemotherapeutic drug and the photosensitizer. The photosensitizer-chemotherapeutic drug is prepared from paclitaxel as a chemotherapeutic drug and coked phaeophytin a as a photosensitizer, a self-assembled nano medicine delivery system is alsoprepared, and synergetic medicine release, in-vivo pharmacokinetics and anti-tumor effects of the drug are evaluated. The predrug is simple in preparation process, small and uniform in nanoparticle size, beneficial to enrichment of nanoparticles at tumor parts through an EPR (Enhanced Permeability and Retention) effect, super large in drug loading capacity and easy in surface modification, and reticuloendothelial system uptaking can be effectively avoided, in addition, uptaking of tumor cells upon the nanoparticles can be effectively improved; while photodynamic treatment is carried out, selective release of medicines can be synergistically triggered in tumor cells; by adopting the 'photosensitizer-chemotherapeutic integration' micromolecule prodrug self-assembled nano drug delivery system designed by the invention, the synergetic anti-tumor effects of the photosensitizer and the chemotherapeutic drug can be remarkably improved.
Owner:SHENYANG PHARMA UNIVERSITY
Arsenic-trioxide-carrying pH-responsive mesoporous silica nanoparticle and preparation method thereof
ActiveCN104644573ANot easy to releaseHas sustained release propertiesPowder deliveryInorganic active ingredientsZeta potentialMesoporous silica
The invention discloses an arsenic-trioxide-carrying pH-responsive mesoporous silica nanoparticle and a preparation method thereof. The preparation method of the arsenic-trioxide-carrying pH-responsive mesoporous silica nanoparticle mainly comprises the following steps: preparing amino modified mesoporous silica by adopting a coprecipitation method, and loading conjugate acid and base ATO and PAA through electrostatic adsorption for preparing PAA-ATO-MSNs. The prepared PAA-ATO-MSNs are circular or quasi-circular in appearance under a transmission electron microscope, average particle size is (158.6+ / -1.32)nm, Zeta potential is (-28.40+ / -0.34)mV, and encapsulation efficiency and drug loading rate are (40.95+ / -3.21)% and (11.42+ / -1.75)% respectively; in vitro drug release is pH-responsive, and accumulated release amount is increased along with reduction of pH value; pharmacokinetic study shows that compared with ATO-Sol and ATO-MSNs, t1 / 2beta of PAA-ATO-MSNs is obviously prolonged and AUC is obviously increased (P is less than 0.01); and PAA-ATO-MSNs in vitro drug release has obvious pH responsiveness and sustained release property, in vivo pharmacokinetic behaviours of a rat can be obviously improved, and the arsenic-trioxide-carrying pH-responsive mesoporous silica nanoparticle carrier can serve as an ATO tumour-targeted drug delivery system and has a good application prospect.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Andrographolide nano suspension and preparation method thereof
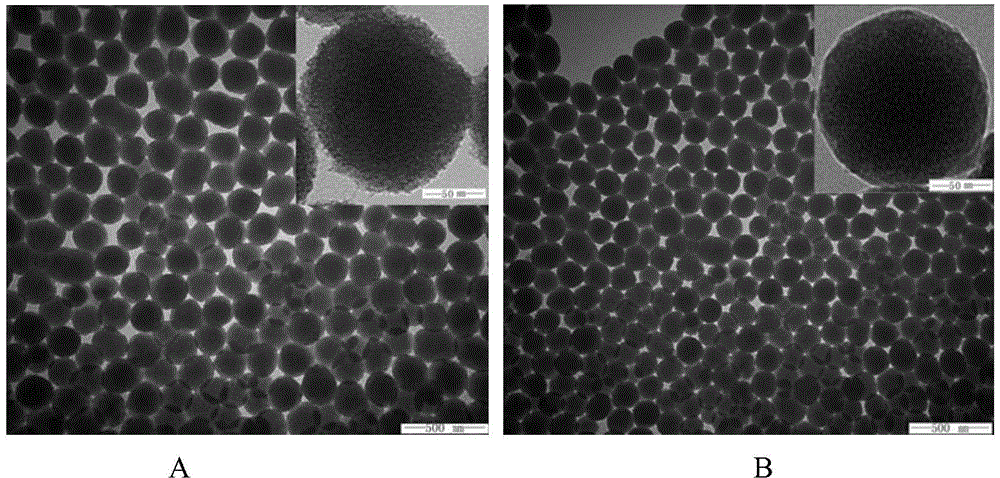
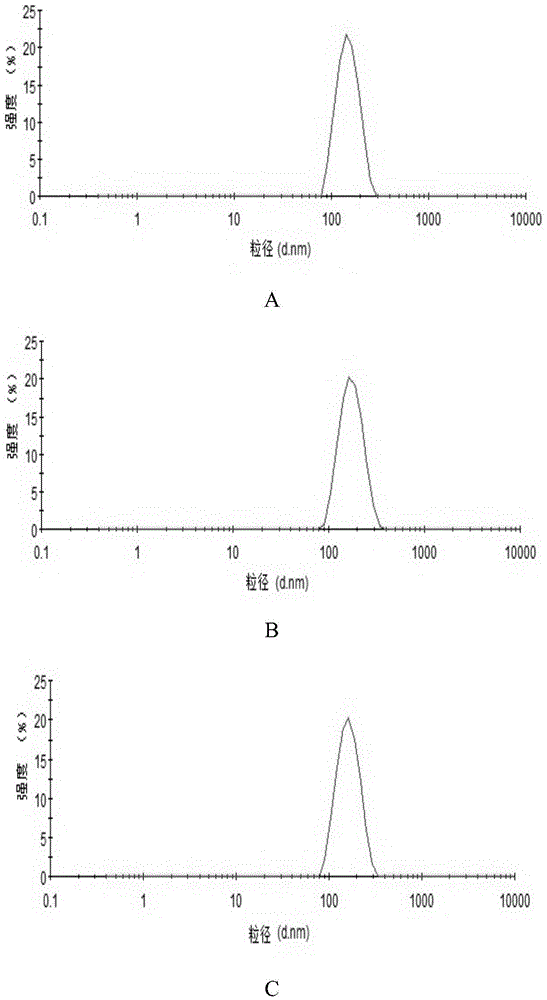
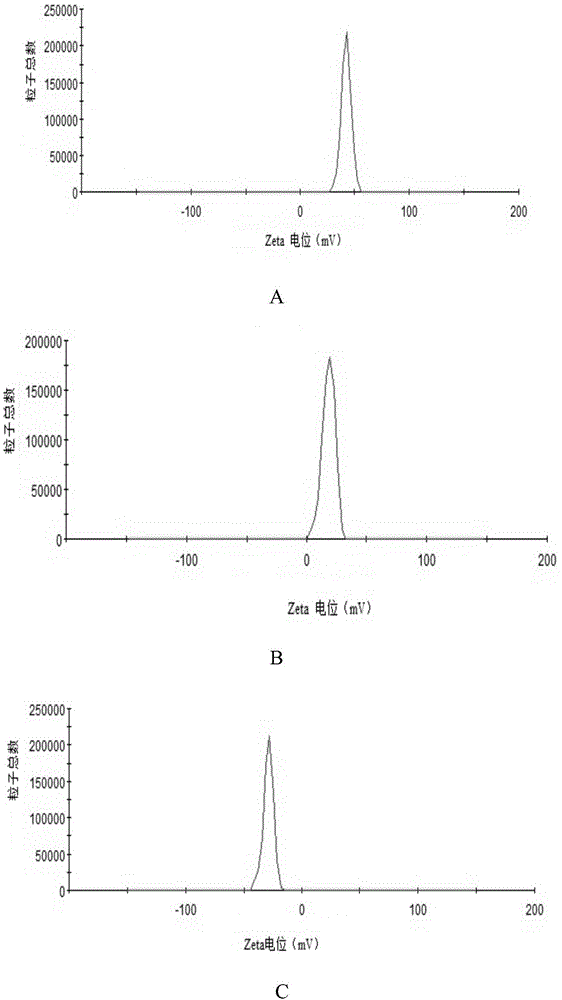
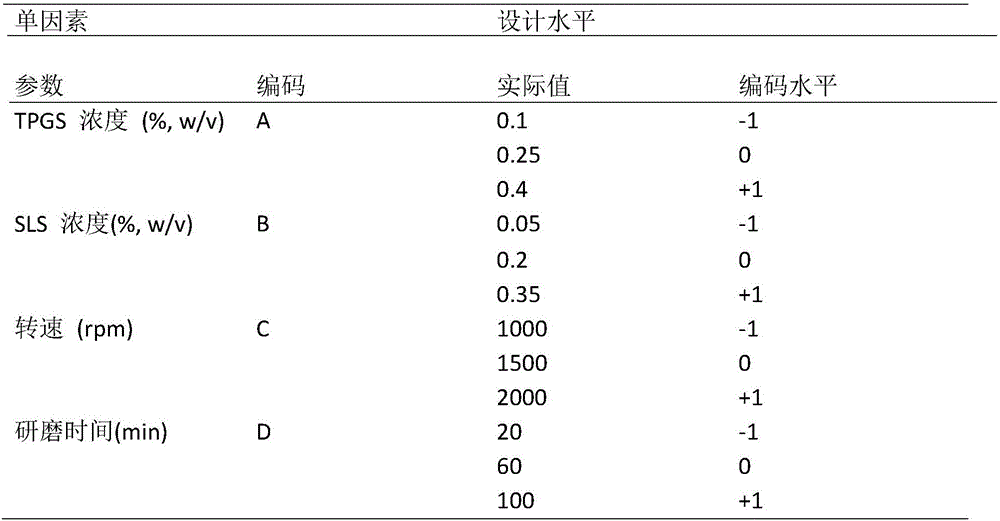
InactiveCN106377500ASmall RDINo significant difference in particle sizeOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The invention belongs to the field of pharmaceutical preparations, and provides an andrographolide nano suspension and a preparation method thereof. The andrographolide has low solubility in water, can be easily excreted by P-gp, has high metabolism rate, and thus, has lower dissolution rate and lower in-vivo bioavailability. In order to enhance the dissolution rate and bioavailability of the drug, a P-gp inhibitor TPGS and an ionic stabilizer SDS are selected to prepare the andrographolide nano suspension by using a medium grinding technique; by using the particle size and distribution as evaluating indicators, a Box-Behnken center combined degisn test optimized preparation technique is utilized to prepare the andrographolide nano suspension; and freeze-drying is used for solidifying. After carrying out the characterization, the research in short-term stability, Caco-2 permeability and in-vitro dissolution, and the in-vivo pharmacokinetic and pharmacodynamic research, the results indicate that the andrographolide nano suspension can increase the dissolution rate of andrographolide and enhance the oral-administration bioavailability and anti-inflammatory action.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Hypoxia improvement-based cisplatin prodrug liposome preparation as well as preparation method and application thereof
ActiveCN106798730AOvercoming tumor hypoxiaEnhanced chemoradiotherapyHeavy metal active ingredientsPeptide/protein ingredientsCisplatinDrug carrier
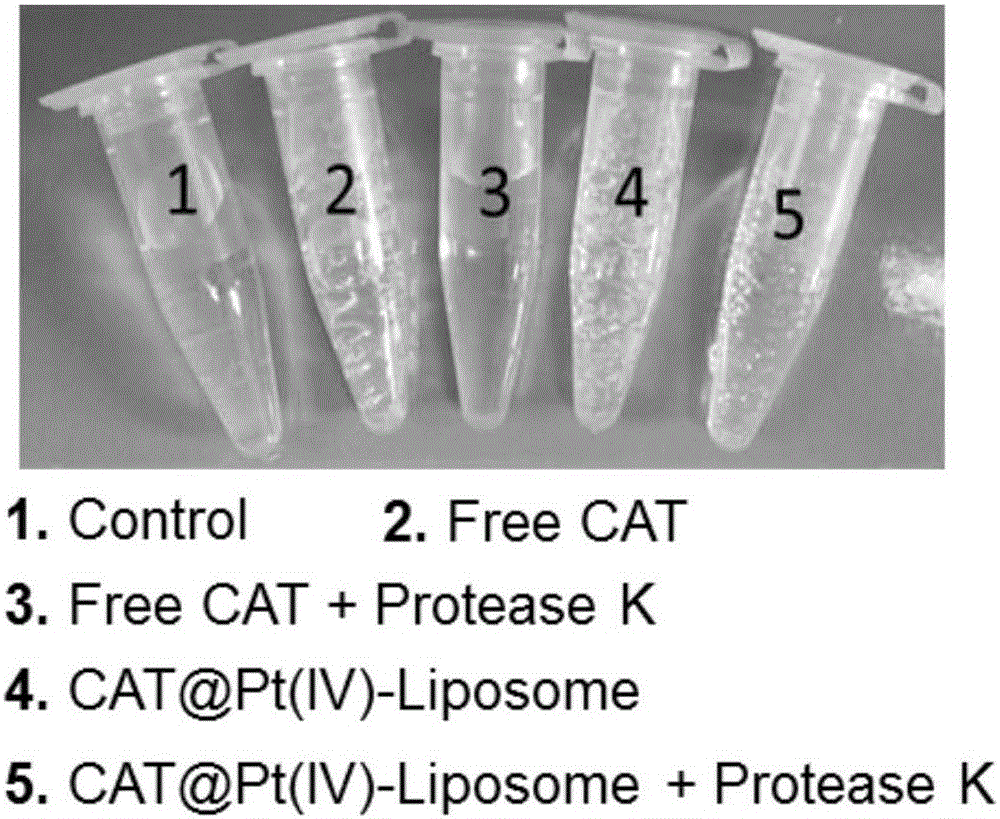
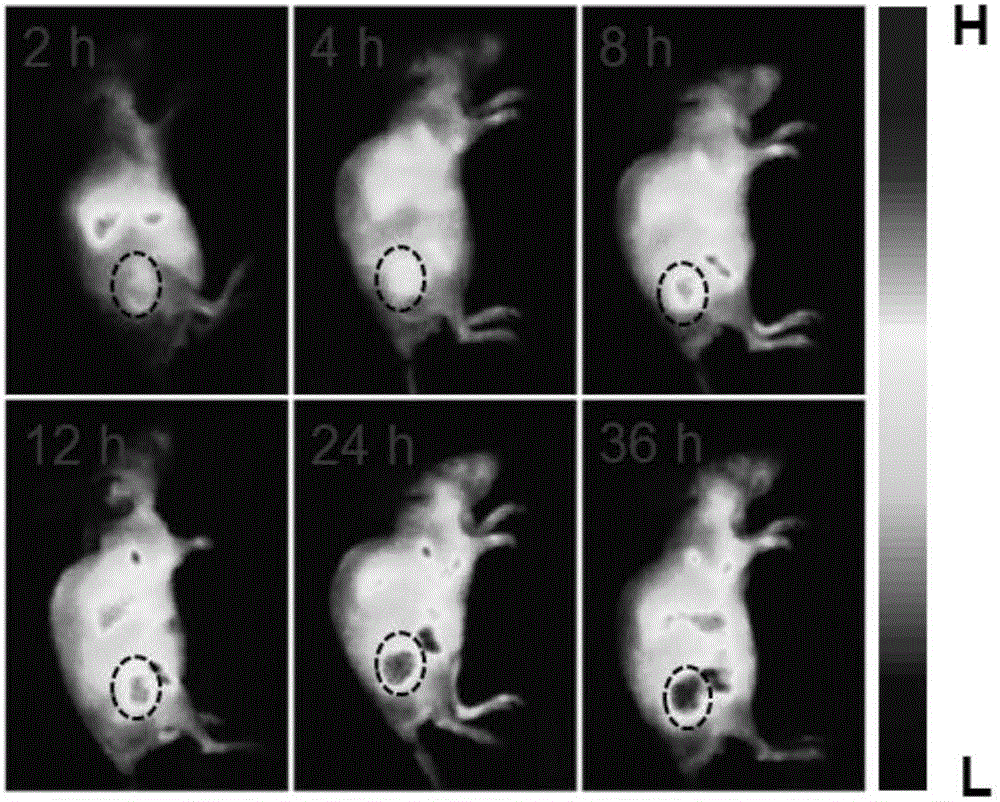
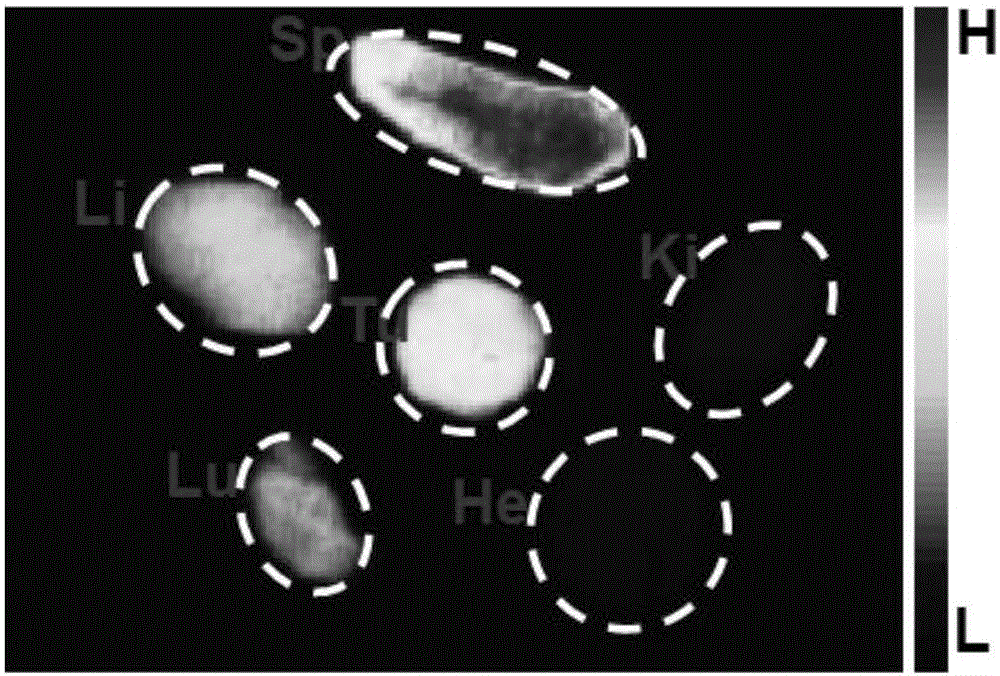
The invention relates to the technical field of biological medicines, and relates to a hypoxia improvement-based radiosensitizing cisplatin prodrug liposome preparation as well as preparation and application thereof, in particular to liposome-cisplatin prodrug-catalase as well as preparation and application thereof, especially application thereof to improvement of hypoxia in a tumor area so as to sensitize radiotherapy in combination with chemotherapy. The liposome preparation provided by the invention mainly comprises a liposome, a cisplatin prodrug and catalase, is uniform in particle size and good in dispersion in water, has the particle size in water mainly concentrating at about 100nm, can simultaneously load photosensitive molecules and the catalase, is an ideal drug carrier, and has good in-vivo pharmacokinetic behaviors. Experiments show that the liposome-cisplatin prodrug-catalase preparation is highly enriched on a tumor site, and can very well improve a hypoxic condition of a tumor environment and increase the oxygen content to sensitize radiotherapy, and through the combination with the chemotherapeutic effect of the cisplatin, a good synergistic tumor-treating effect is ultimately achieved.
Owner:JE & NA BIOTECH CO LTD
Metformin hydrochloride osmotic pump controlled release tablet and preparation method thereof
ActiveCN105878204AImproved controlled releaseGood compressibilityOrganic active ingredientsMetabolism disorderRelease modulatorAdhesive
The invention relates to the field of pharmaceutical preparations and particularly provides a controlled release tablet of metformin hydrochloride and a preparation method of the controlled release tablet. The controlled release tablet provided by the invention contains a tablet core, an insoluble semi-permeable membrane and a drug release micro-pore, wherein the tablet core contains metformin hydrochloride, an adhesive, a release regulator, an absorption accelerant and a lubricating agent. The metformin hydrochloride in the controlled release tablet provided by the invention can be stably released at a constant speed, so that absorption of drugs is more facilitated, and a better in vivo pharmacokinetic curve is realized. Moreover, the preparation method of the controlled release tablet is simple and easy in process, and the technical defect of poor compressibility of the tablet is solved.
Owner:HEFEI LIFEON PHARMA
Preparation containing bendopa methyl ester and preparation method thereof
The invention discloses a preparation containing L-dopa methyl ester and a preparation method thereof. The preparation method comprises the following steps of: adding L-dopa methyl ester into a melted carrier material such as polyethylene glycol (PEG), poloxamer 188 or hydroxypropyl cellulose (HPC), stirring at high speed to uniformly disperse drug particulates into the carrier material, making into solid dispersion, and further making into capsule, tablet or granule containing 50-500mg L-dopa methyl ester, as required. The preparation increases the dispersion degree of L-dopa methyl ester and reduces the local gastrointestinal concentration thereof, so as to reduce the side effects thereof; and at the same time, the absorption of L-dopa methyl ester is improved, so that the in vivo pharmacokinetic curve is more stable. The L-dopa methyl ester solid dispersion is a new preparation for treating Parkinson's disease.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD +1
Analysis method for four anthraquinones in blood plasma and application of four anthraquinones in pharmacokinetics
ActiveCN103575830AThe test result is accurateHigh sensitivityComponent separationMonomethyl etherBlood plasma
The invention discloses an analysis method for determining rheum emodin, aloe-emodin, chrysophanol and emodin monomethyl ether in blood plasma at the same time and application of rheum emodin, aloe-emodin, chrysophanol and emodin monomethyl ether in pharmacokinetics, wherein the analysis method for determining rheum emodin, aloe-emodin, chrysophanol and emodin monomethyl ether in the blood plasma at the same time comprises the following steps: (1) preparing a sample; (2) detecting. The analysis method for determining rheum emodin, aloe-emodin, chrysophanol and emodin monomethyl ether in the blood plasma at the same time has good specificity, high precision degree, high accuracy and wide linear range and can be used for in vivo pharmacokinetic determination of Chinese medicinal compositions.
Owner:北京中一堂科技有限公司
Polysialic acid lipid grafted derivatives and applications thereof
ActiveCN106554425AThe degree of polymerization is controllableExtend cycle timePowder deliveryPharmaceutical non-active ingredientsLipid formationSialic acid
The invention belongs to the field of medicine preparations, and particularly relates to polysialic acid lipid grafted derivatives, a preparing method thereof and applications of the derivatives, particularly applications for preparation and modification of microparticle preparations. Sialic acid units in the polysialic acid are connected through alpha-2,8-glucosidic bonds, and lipid segments and hydroxy in the sialic acid units are connected through ester bonds. A structural formula is shown in the description, wherein SA is a sialic acid unit, x is the number of the lipid segments grafted in the polysialic acid molecule, m is the number of the sialic acid units in the polysialic acid molecule, the m is not more than 100 and not less than 1, the x is not more than 30 and not less than 1, x / m is 5-30%, R-CO- is derived from R-COOH, and the R-COOH is a lipid compound containing carboxyl. Immunogenicity of microparticle preparations prepared or modified by the compounds is extremely low and the microparticle preparations have excellent in-vivo pharmacokinetic properties.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation of carbon nanotube doped fenbufen molecularly imprinted polymer sustained-release material
ActiveCN106860399AEasy to operateEasy to makeOrganic active ingredientsAntipyreticTetrafluoroboratePolymer science
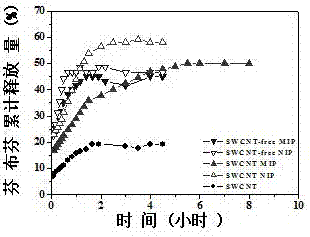
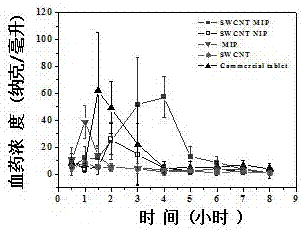
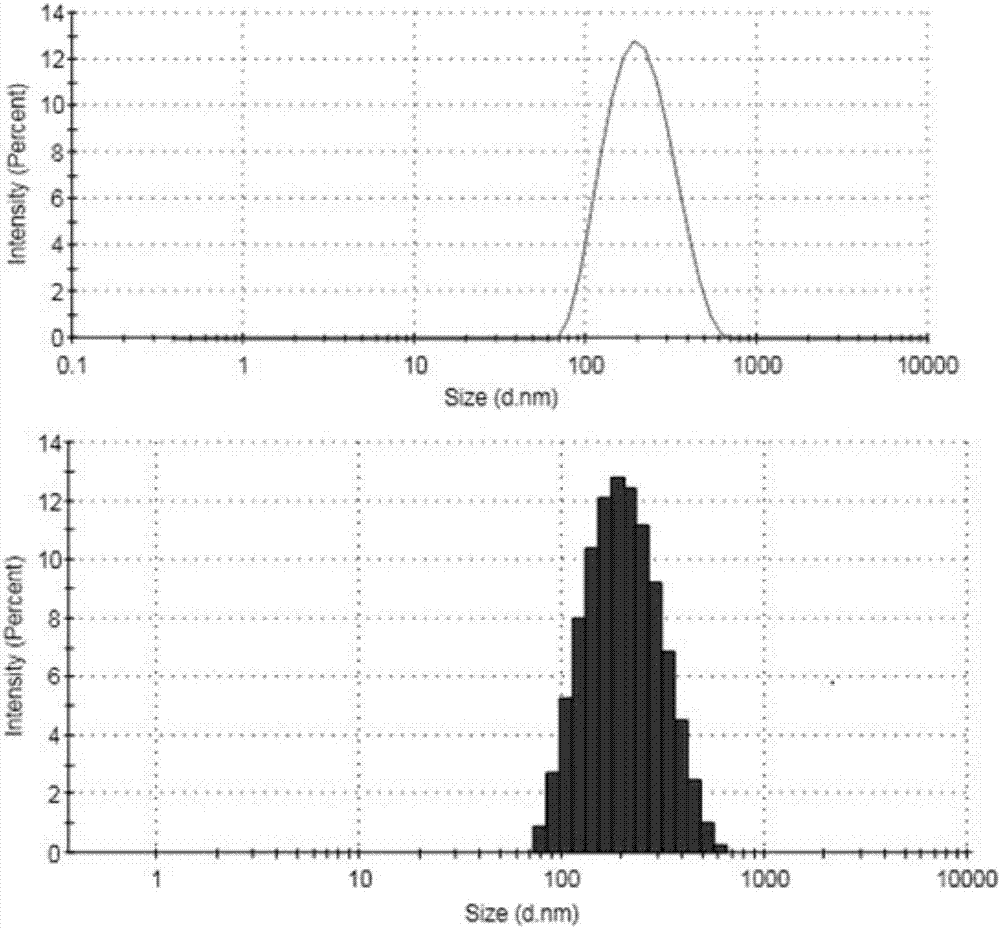
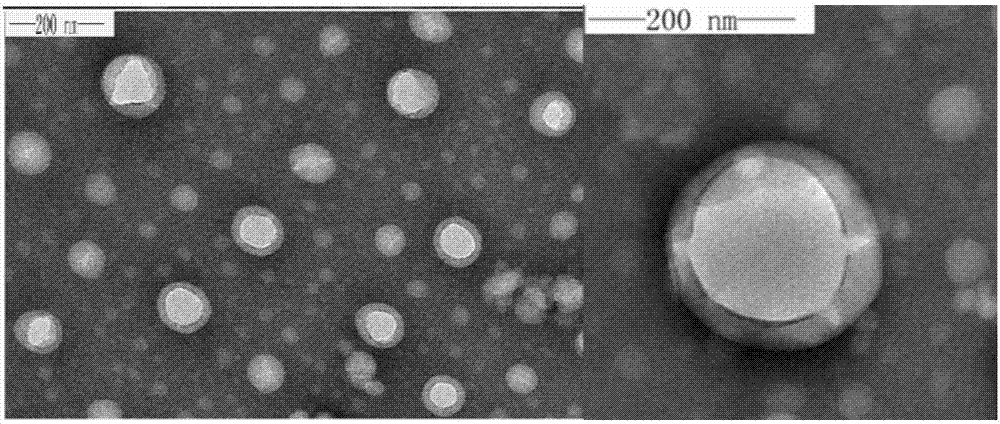
The invention relates to preparation of a carbon nanotube doped fenbufen molecularly imprinted polymer sustained-release material and specifically relates to a fenbufen sustained-release molecularly imprinted polymer material which is prepared by utilizing ionic liquid 1-butyl-3-methylimidazole tetrafluoroborate and a deep-eutectic solvent as a binary pore-foaming agent and doping single-wall carbon nanotubes. The fenbufen sustained-release molecularly imprinted polymer material is prepared from raw materials by mass: 0.67 to 0.76% of fenbufen, 0.03 to 0.06% of single-wall carbon nanotube, 2.41 to 2.75% of 4-vinyl pyridine, 18.16 to 20.69% of ethylene glycol dimethacrylate, 18.37 to 48.39% of deep-eutectic solvent, 30.06 to 57.08% of 1-butyl-3-methylimidazole tetrafluoroborate and 0.25 to 0.29% of azodiisobutyronitrile. The fenbufen sustained-release molecularly imprinted polymer material disclosed by the invention has a good sustained-release effect; in-vivo pharmacokinetics show that compared with 1.5h peak time and 46.1% bioavailability of an imprinted polymer without carbon nanotubes, peak time of blood drug concentration in a rat body can reach 4 hours and bioavailability can be 140% compared with commodity medicine, and performance is stable. The preparation of the carbon nanotube doped fenbufen molecularly imprinted polymer sustained-release material disclosed by the invention lays a foundation for a molecularly imprinted technology to be applied to a medicine transfer system.
Owner:TIANJIN MEDICAL UNIV
Polymeric micelle capable of realizing synchronous administration of sorafenib and curcumin and preparation method of polymeric micelle
ActiveCN107049944ARealize combination medicineSmall toxicityKetone active ingredientsPharmaceutical non-active ingredientsSolubilityTreatment effect
The patent of the invention relates to a polymeric micelle capable of realizing synchronous administration of sorafenib and curcumin and a preparation method of the polymeric micelle, and belongs to the field of novel medicine preparations in health and medicines. The PCL-PEG-PCL (polycaprolactone -polyethylene glycol-polycaprolactone) is taken as a carrier material, the polymeric micelle capable of synchronously releasing sorafenib and curcumin is prepared according to a filming rehydration method, the solubility and the bioavailability of sorafenib and curcumin are improved, and the purposes of synergetic administration of medicines and enhancement of treatment effects are fulfilled. The patent concretely investigates the optimal polymer carrier material, the carrier material, the medicine dosage ratio, the rehydration temperature, time and volume of the filming rehydration method, the in vitro dissolution and the studies on in vivo pharmacokinetics of mice, the finally prepared polymeric micelle can realize synchronous administration of sorafenib and curcumin, the long-circulation effect is obvious, and the polymeric micelle has broad research significances and commercial popularization prospect.
Owner:LIAONING UNIVERSITY
Blank high-molecular microspheres and preparation method thereof
InactiveCN106474071AGood compatibilityAvoid rapid degradationGranular deliveryMacromolecular non-active ingredientsControlled releaseMicrosphere
The invention provides blank high-molecular microspheres prepared by a spray drying method. With use of the instant drying ability of spray drying and the excellent dissolving ability of dichloromethane and the like, a high-molecular material is made into the microspheres. Through the characterization observation and particle size determination of the microspheres, the diameter size and surface appearance of a slow-release carrier support material are known, and the diameter of the blank high-molecular microspheres is in line with the size for injection. Compared with other microsphere preparation methods, the spray drying method is simple, the repetition rate is high, and the structure of the microspheres is clear. Animal in-vivo pharmacokinetics and in-vitro release degree evaluation show that the microsphere carrier is used as a good sustained-release carrier for different treatment drugs in the field of drug sustained or controlled release.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
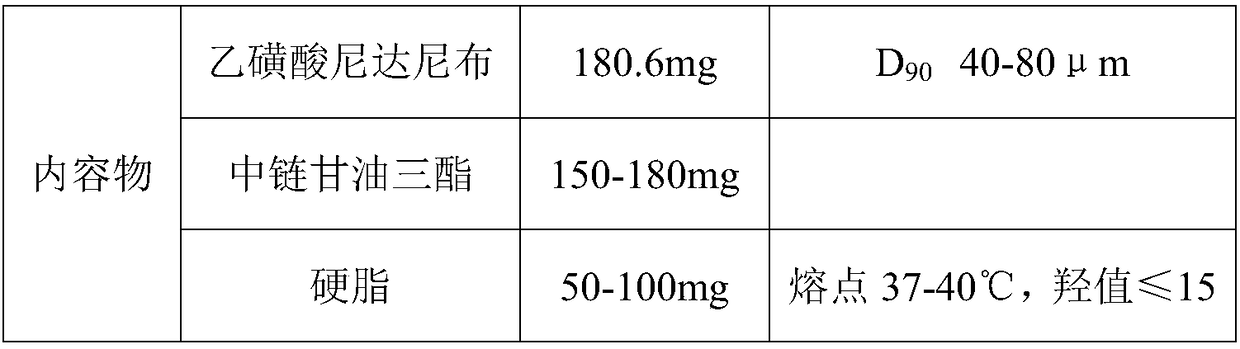
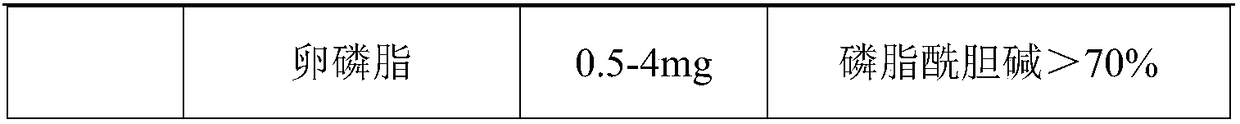
Soft nintedanib ethanesulfonate capsule and preparation method thereof
InactiveCN108078952AGreat physical stabilityThe bioavailability has a large impact onOrganic active ingredientsPharmaceutical non-active ingredientsMedicineBioavailability
The invention belongs to the technical field of medicines, and particularly relates to a preparation method of a content of a soft nintedanib ethanesulfonate capsule. The content is prepared from nintedanib ethanesulfonate, wherein the grain size distribution range of the nintedanib ethanesulfonate is D90 from 40 mu m to 80 mu m. By studying the stability of a suspension system of the content of the soft nintedanib ethanesulfonate capsule and determining in-vivo pharmacokinetic parameters of wistar rats, the influence of the grain size of the nintedanib esylate as a raw material on the physical stability and bioavailability of the soft nintedanib ethanesulfonate capsule is discovered to be great, and the soft nintedanib ethanesulfonate capsule is ultimately discovered to have good physicalstability and be similar to pharmacokinetic behaviors of reference preparations in terms of absorption when the grain size of the nintedanib ethanesulfonate material is 40 mu m to 80 mu m, and moreover, since the grain size control range of the raw material is broadened, the production cost and the technological requirements are reduced. The invention further provides the preparation method of the content, the process of which is reasonable.
Owner:REYOUNG PHARMA
Hypoxia responsive liposome preparation, preparation method and application thereof
ActiveCN106562930AEfficient and coordinated killingEfficient enrichmentEnergy modified materialsAmine active ingredientsPhotodynamic therapyMedicine
The invention relates to the technical field of biomedicines and relates to a hypoxia responsive liposome preparation, and preparation and applications thereof. In particular, the invention relates to hypoxia responsive liposome-AQ4N-dihydroporphin e6 and preparation and application thereof, and especially, application thereof in enhancement of killing cells in tumor hypoxia zones in photodynamics therapy. The liposome preparation is composed of a liposome, AQ4N and dihydroporphin e6, has uniform particle size and is excellent in dispersion in water. The particle size of the liposome preparation is mainly about 100 nm in water. The liposome preparation can carry photosensitive molecules and dimethyldiguanide and is an ideal medicine carrier, and also has excellent in-vivo pharmacokinetics behavior. A test proves that the liposome-AQ4N-dihydroporphin e6 preparation is very-highly enriched at tumor parts, so that after the photodynamics therapy, the preparation can cause a more serious hypoxia status in the tumor zone, thereby improving the killing effect on the hypoxia cells of the AQ4N and obtaining excellent synergistic therapy effects on tumor.
Owner:SUZHOU INNOVATIVE BIOMATERIALS & PHARM CO LTD
Ligustrazine-butyphthalide combination compound, preparation method of ligustrazine-butyphthalide combination compound, and application of ligustrazine-butyphthalide combination compound to pharmaceuticals
ActiveCN106928155AExtended half-lifeImprove bioavailabilityOrganic active ingredientsNervous disorderAdjuvantInduced platelet aggregation
The invention discloses a ligustrazine-butyphthalide combination compound, a preparation method of the ligustrazine-butyphthalide combination compound and an application of the ligustrazine-butyphthalide combination compound to pharmaceuticals. The ligustrazine-butyphthalide combination compound has the following structural formula I as in the description. The phthalic anhydride and the ligustrazine are taken as starting materials, bromization, nucleophilic addition, catalytic dehydration, ester hydrolysis, reduction and esterification reactions are conduced to prepare the ligustrazine-butyphthalide combination compound; a pharmaceutical composition takes the ligustrazine-butyphthalide combination compound as an active pharmaceutical ingredient and is a pharmaceutically acceptable carrier, excipient, diluent, adjuvant, intermedium or a composition. The prepared ligustrazine-butyphthalide combination compound has an excellent effect of inhibiting in-vitro platelet aggregation (ADP) induced platelet aggregation, has a better in-vivo pharmacokinetics properties, and can be used for preventing and treating cardiovascular and cerebrovascular diseases, vascular senile dementia and the complications.
Owner:GUIZHOU MEDICAL UNIV
Analysis method for five flavonoid glycosides in blood plasma and application of five flavonoid glycosides in pharmacokinetics
ActiveCN103575820AThe test result is accurateHigh sensitivityComponent separationNaringinBlood plasma
The invention provides a method for quantitatively detecting naringin, aurantiamarin, neohesperidin, baicalin and wogonoside in a blood plasma sample by adopting liquid chromatography tandem mass spectrometry, and the method comprises the following steps: (1) preparaing a sample; (2) detecting. The analysis method provided by the invention has good specificity, high precision degree, high accuracy and wide linear range and can be used for in vivo pharmacokinetic determination of Chinese medicinal compositions.
Owner:北京中一堂科技有限公司
Long acting injectable crystal formulations of estradiol metabolites and methods of using same
The present invention provides sustained release formulations of estradiol metabolites whereby the in vivo pharmacokinetics are manipulated by a method selected from the group consisting of chemical modification, crystal packing formation, particle size or a combination thereof. Such compositions are useful in the long-term treatment of a wide variety of diseases.
Owner:PR药品有限公司
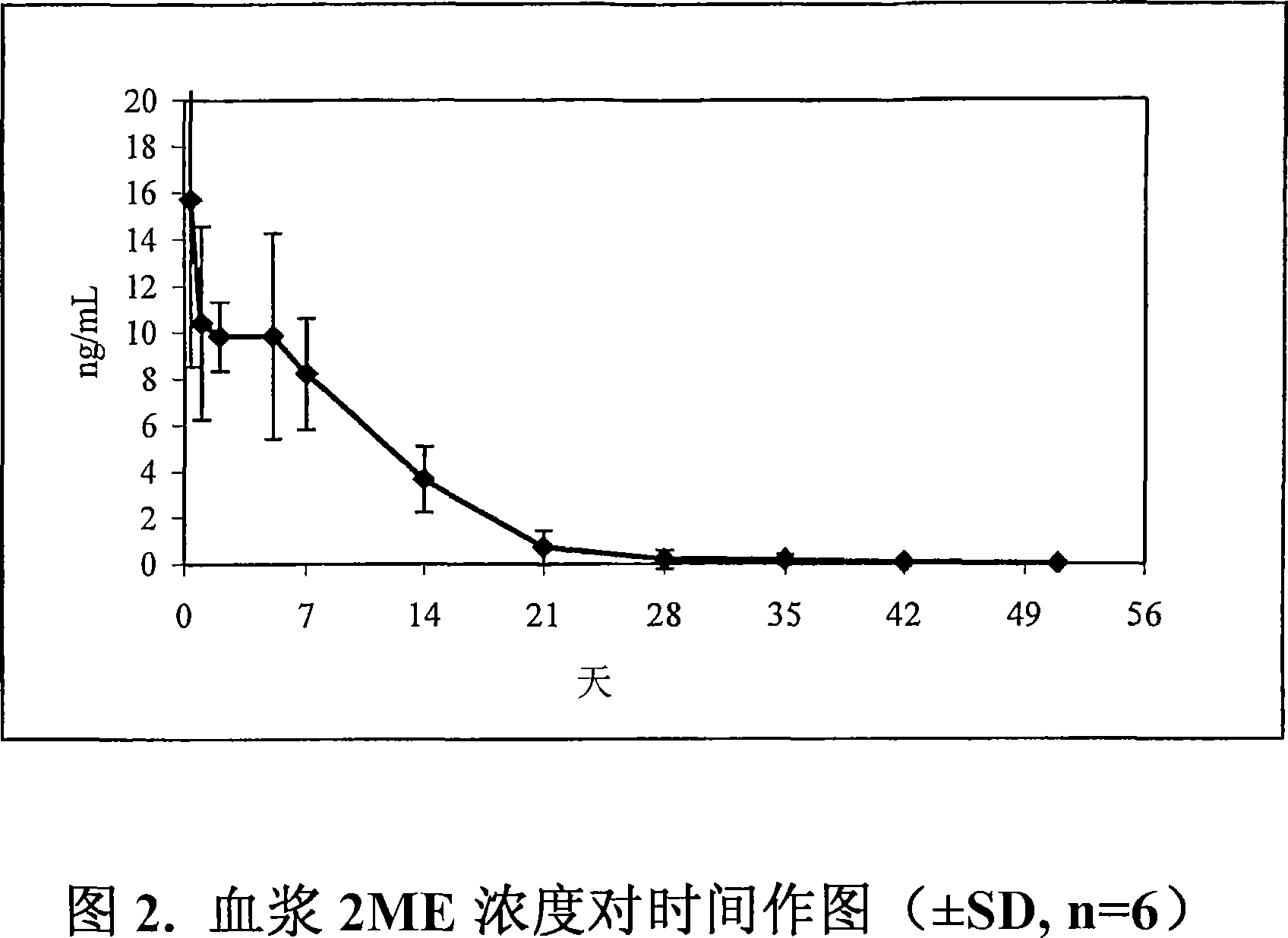
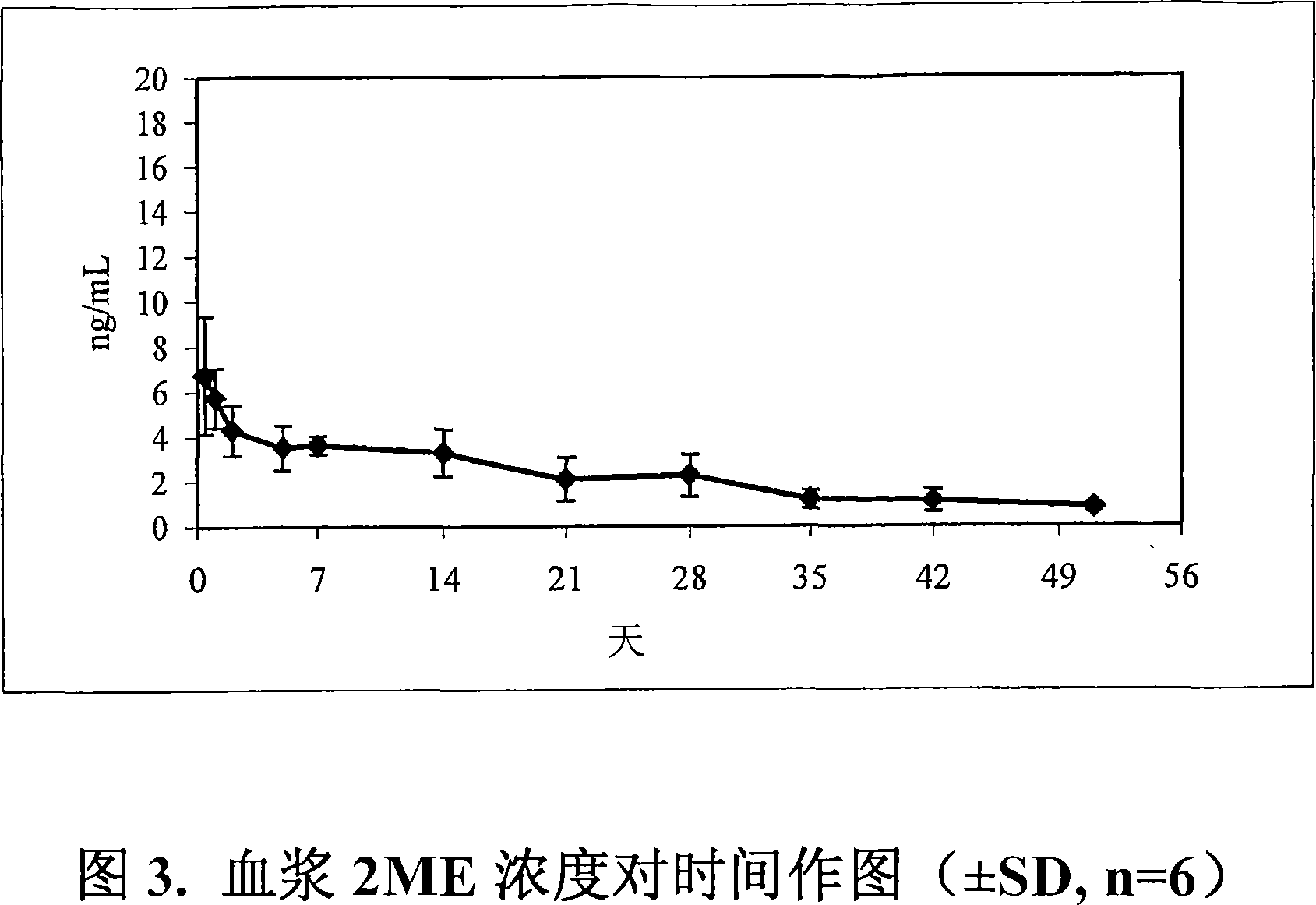
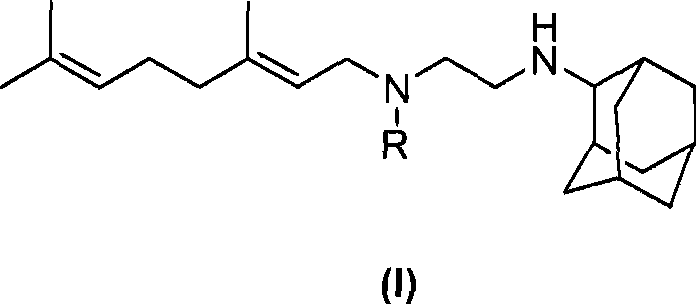
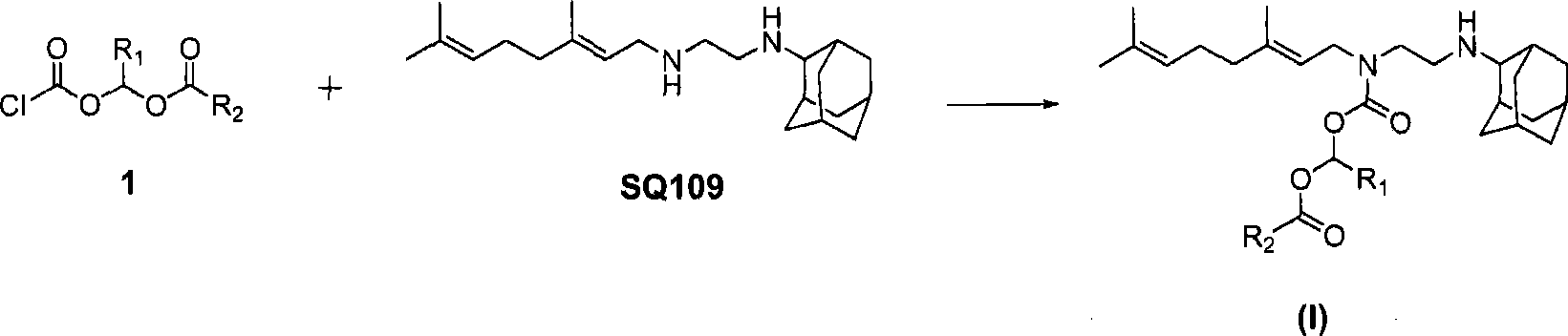
Novel ethylene diamine derivative
InactiveCN101468958AGood pharmacokinetic propertiesImprove utilizationAntibacterial agentsCarbamic acid derivatives preparationEthylene diamineStructural formula
The invention relates to ethylenediamine compounds of which the structural formula is as shown in a formula (I), pharmaceutically acceptable salts, a preparation method thereof and application of the ethylenediamine compounds in preparing medicines for treating infectious diseases caused by tubercle bacillus and particularly infectious diseases caused by multidrug resistant tubercle bacillus. The compounds of which the structural formula is shown in the (I) have good in vitro tubercle bacillus resistant activity and good in vivo pharmacokinetics property.
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD
4-pyridine substituted phthalazinone compound as well as preparation method, pharmaceutical composition and application thereof
ActiveCN112125881AGood liver targetingOrganic active ingredientsGroup 5/15 element organic compoundsPharmaceutical drugVirus inhibitors
The invention relates to a 4-pyridine substituted phthalazinone compound, a preparation method, a pharmaceutical composition and an application thereof, the structure of the 4-pyridine substituted phthalazinone compound is shown as a formula I, the compound of the formula I has improved pharmaceutical physicochemical properties and in-vivo pharmacokinetic properties, oral bioavailability is high,druggability is good, The compound is a non-nucleoside small-molecule HBV virus inhibitor which is novel in structure and can be orally taken.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Method for evaluating in-vivo and in-vitro correlation of diclofenac sodium sustained release tablets
The invention discloses a method for evaluating in vivo and in vitro correlation (In Vivoin Vitro Correlation) of a diclofenac sodium sustained release tablet, and a novel diclofenac sodium sustained release tablet. According to the method for evaluating the in-vivo and in-vitro correlation of the diclofenac sodium sustained release tablet, the in-vivo pharmacokinetic characteristics can be well evaluated. The formula of the diclofenac sodium sustained release tablet comprises the following components: 100 mg / tablet of diclofenac sodium, 119 mg / tablet of cane sugar, 59 mg / tablet of hexadecanol, 4 mg / tablet of povidone K30, 3 mg / tablet of colloidal silicon dioxide, 3 mg / tablet of magnesium stearate and 10 mg / tablet of film coating premix. According to the diclofenac sodium sustained-release tablet disclosed by the invention, the release speed of the medicine is controlled, the irritation of the traditional diclofenac sodium sustained-release tablet to the stomach is reduced, and meanwhile, the administration mode of keeping a relatively high effective blood concentration for a long time is achieved. The medicine effect is ensured, the side effect is reduced, and the clinical use is greatly facilitated.
Owner:HQ PHARMA (SHANGHAI) CO LTD
Pharmaceutical preparation
InactiveCN102316856APrevent pharmacological effects from decreasingPrevent pharmacological effects from risingMetabolism disorderPharmaceutical non-active ingredientsImmediate releaseBULK ACTIVE INGREDIENT
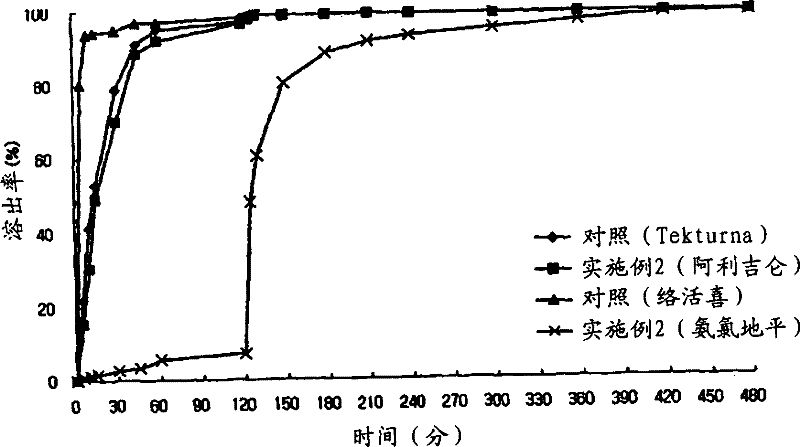
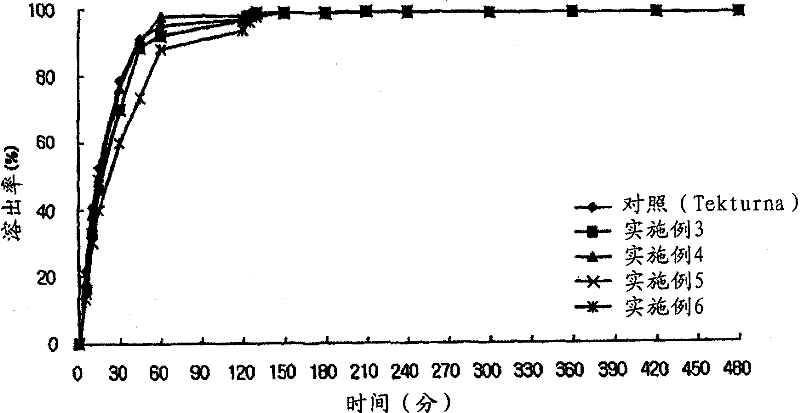
The present invention relates to a pharmaceutical preparation comprising an immediate-release compartment containing a renin inhibitor as a pharmacologically active ingredient, and an extended-release compartment containing a dihydropyridine calcium channel blocker as a pharmacologically active ingredient. The pharmaceutical preparation of the present invention can avoid in vivo pharmacokinetic interaction between the renin inhibitor and the dihydropyridine calcium channel blocker, induces optimum pharmacological effects in accordance with the in vivo absorption performance of each of the active ingredients, and enables drugs to be released at the time period in which each of the active ingredients exhibits the pharmacological effects thereof, to thereby increase clinical effects, and can thus be valuably used in the prevention or treatment of metabolic syndrome, cardiovascular disease, and kidney disease.
Owner:HANALL PHARMA CO LTD
Paliperidone Implant Formulation
ActiveUS20190328654A1Organic active ingredientsPharmaceutical delivery mechanismGlycolic acidPharmaceutical drug
An injectable intramuscular depot composition suitable for forming an in situ solid implant in a body, comprising a drug which is paliperidone and / or its pharmaceutical acceptable salts in any combination thereof, a biocompatible copolymer based on lactic and glycolic acid having a monomer ratio of lactic to glycolic acid of about 50:50 and DMSO as solvent, wherein the composition releases the drug with an immediate onset of action and continuously for at least 8 weeks and wherein the composition has a pharmacokinetic profile in vivo suitable for the formulation to be administered each 8 weeks or even longer periods.
Owner:LAB FARM ROVI SA
1,2,4-Triazolone Derivative
The present invention provides a 1,2,4-triazolone derivative represented by Formula (1A) having an antagonistic activity on the arginine-vasopressin 1b receptor or a pharmaceutically acceptable salt thereof and provides a pharmaceutical composition comprising the compound or the salt as an active ingredient, in particular, a therapeutic or preventive agent exhibiting favorable pharmacokinetics in a disease such as mood disorder, anxiety disorder, schizophrenia, Alzheimer's disease, Parkinson's disease, Huntington's chorea, eating disorder, hypertension, gastrointestinal disease, drug addiction, epilepsy, cerebral infarction, cerebral ischemia, cerebral edema, head injury, inflammation, immune-related disease, or alopecia.
Owner:TAISHO PHARMACEUTICAL CO LTD
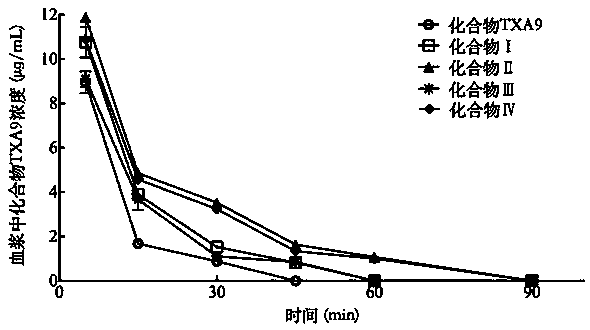
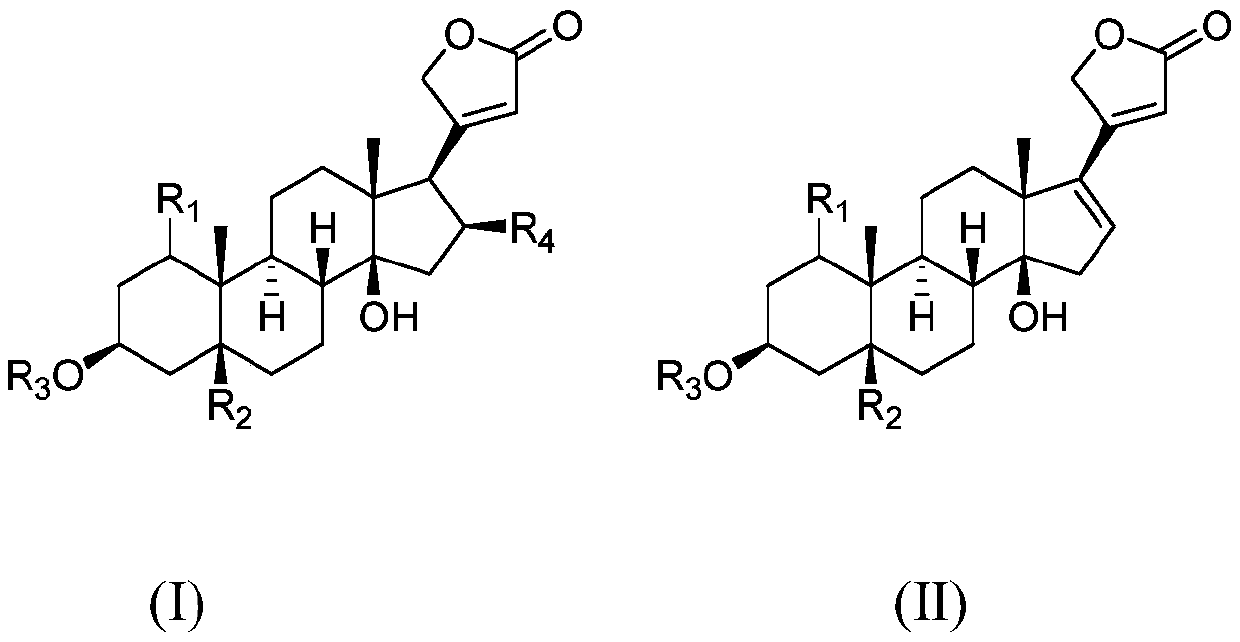
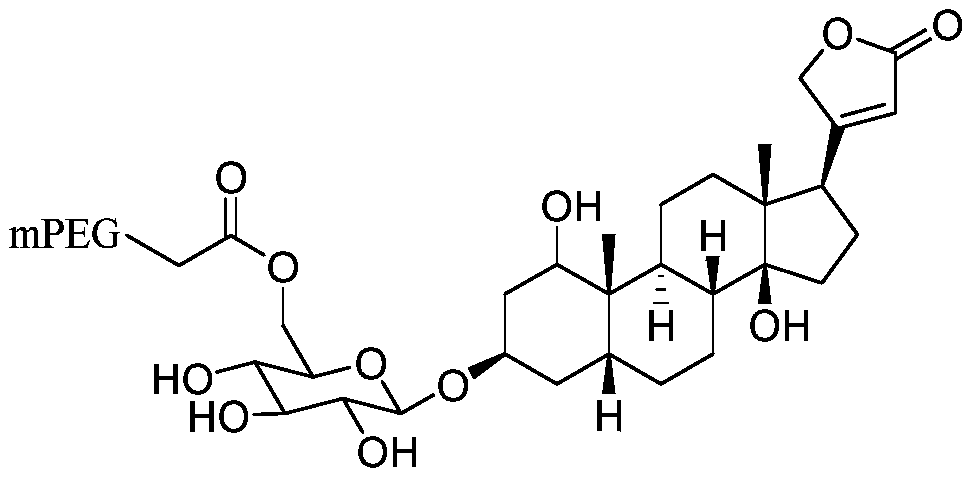
Polyethylene glycol modified cardiac glycoside compound prodrug and anti-tumor application thereof
ActiveCN110772644AStrong hydrophilic groupGood water solubilityOrganic active ingredientsPharmaceutical non-active ingredientsDrug administrationDrug efficiency
The invention belongs to the technical field of medicine, and relates to a polyethylene glycol modified cardiac glycoside compound prodrug and a preparation method thereof, a drug composition including the compound prodrug, and application of the compound prodrug and the drug composition to preparation of an anti-tumor drug. The water solubility of a prototype drug is significantly improved by theprodrug, and the problem of difficult drug administration of the prototype drug is solved. An in-vitro cell experiment shows that the prodrug has the good effect of inhibiting growth of tumor cells.In-vivo pharmacokinetic property study shows that the prodrug can prolong the in-vivo half-life period. In-vivo pharmaceutical effect evaluation of nude mice shows that the prodrug had a good growth inhibition effect on a transplanted tumor of a human lung cancer A549 cell strain inoculated in the nude mice, the inhibition intensity of the prodrug is significantly better than that of the prototypedrug, and the better antitumor effect is achieved. The structure of the prodrug is as follows (please see the specifications for the structure), wherein R1, R2, R3 and R4 are as described in the claims and the specification.
Owner:SHENYANG PHARMA UNIVERSITY
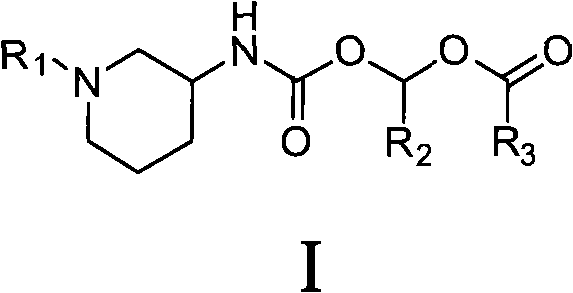
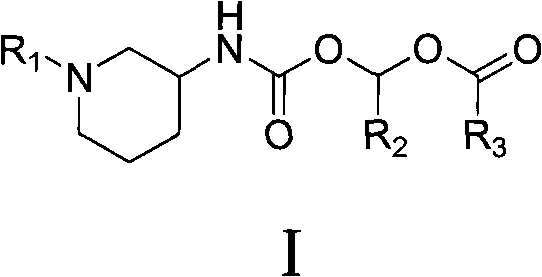
Piperidine carbamic acid ester derivative and application thereof
InactiveCN101735218ABlood sugarImprove oral bioavailabilityOrganic chemistryMetabolism disorderDiseaseIGT - Impaired glucose tolerance
The invention provides a piperidine carbamic acid ester compound shown as the general formula I and possible isomers, medicinal salts, crystal forms, hydrates, solvates and medical compositions thereof. The invention also relates to applications of the possible isomers, the medicinal salts, the crystal forms, the hydrates, the solvates and the medical compositions in preparing medicaments for treating and / or preventing diseases related to DPP-IV, such as diabetes mellitus, particularly for non-insulin-dependent diabetes mellitus and impaired glucose tolerance, and the applications have good property of in vivo pharmacokinetics.
Owner:廖国超
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com