Ligustrazine-butyphthalide combination compound, preparation method of ligustrazine-butyphthalide combination compound, and application of ligustrazine-butyphthalide combination compound to pharmaceuticals
A technology of ligustrazine and butylphthalide, applied in the field of Ligustrazine-butylphthalide combination compound and its preparation, can solve the problems of short half-life, poor fat solubility, poor water solubility, etc., to prolong half-life, improve bioavailability, The effect of improving fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
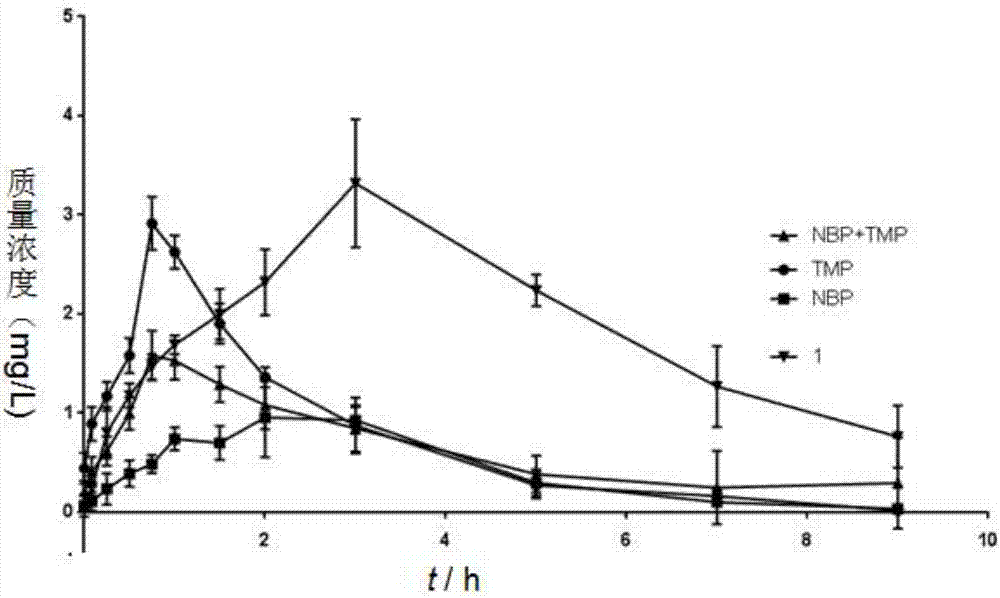
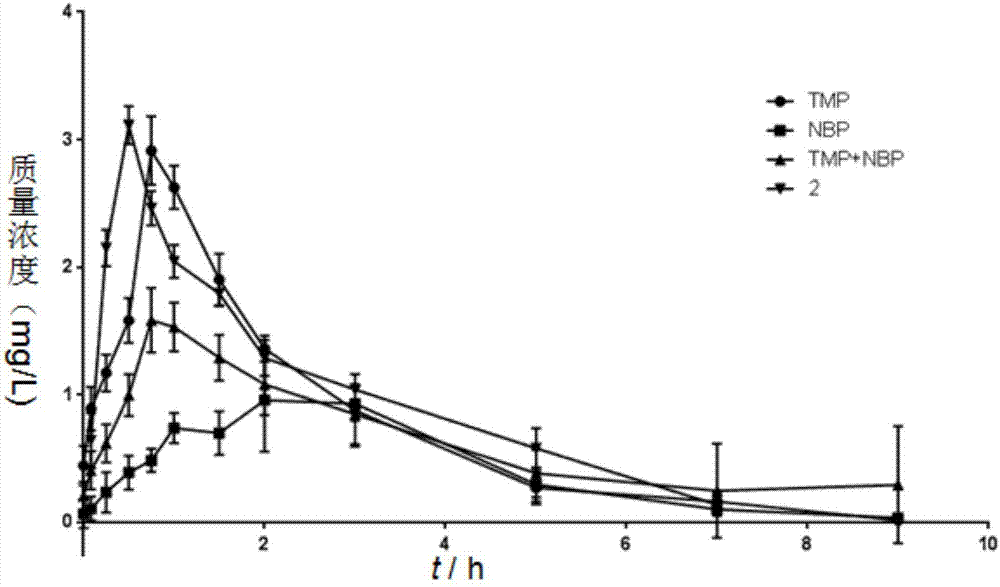
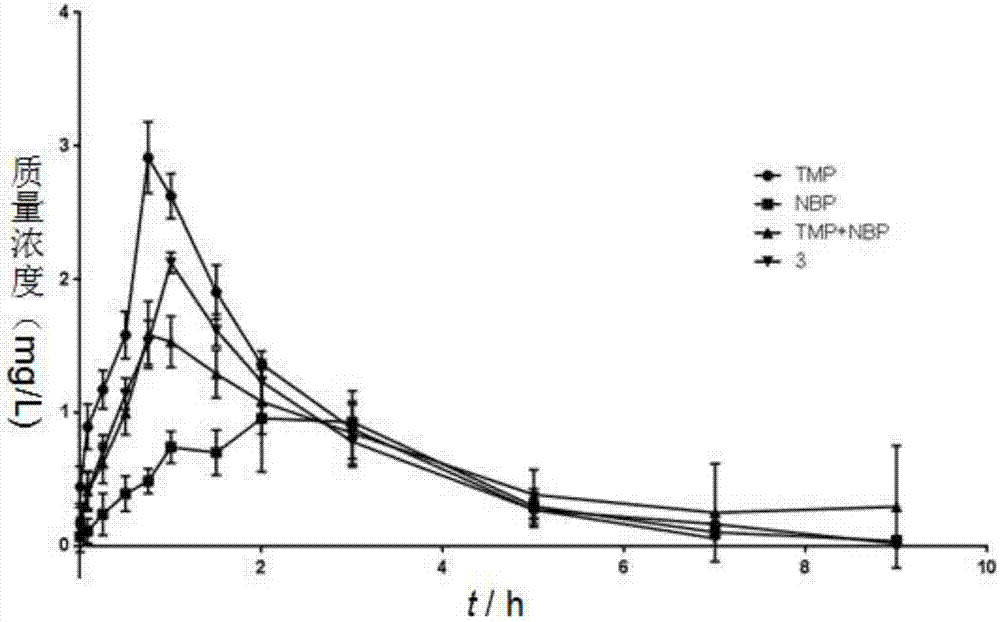
[0027] The preparation method of ligustrazine-butylphthalide complex compounds of the present invention comprises the following steps: using phthalic anhydride and ligustrazine as starting materials, through NBS free radical bromination, n-butyllithium nucleophilic addition , P-toluenesulfonic acid catalytic dehydration, ester hydrolysis under alkaline conditions, Pd / C plus H 2 Reduction, esterification reaction, Ligustrazine - butylphthalide splicing compounds were obtained. The specific reaction of synthetic target compound 1, 2, 3 is as follows:
[0028]
[0029] Synthetic routes of target compounds 1, 2, 3
Embodiment 1
[0031] The preparation of (3,5,6-trimethylpyrazin-2-yl) methyl-2-pentanoyl benzoate (1) comprises the following steps:
[0032] (1) Synthesis of 2-bromomethyl-3,5,6-trimethylpyrazine (4): Add 20.0g (348mmol) TMP, 18.2g (103mmol) N-bromosuccinate in a 250mL round bottom flask imide (NBS), with 80mL CCl 4 After dissolving the raw material, irradiate with a 60W incandescent lamp and reflux for 12 hours. After TLC detects that the reaction is complete, filter it with suction and evaporate the filtrate to obtain the crude product, which is purified by silica gel column chromatography with V (petroleum ether): V (ethyl acetate) = 12:1 to obtain 15.4 g of white solid, 70% yield, m.p.41-42°C. 1 HNMR (CDCl 3 ,400MHz), δ:4.55(s,2H),2.58(s,3H),2.51(s,3H),2.50(s,3H);HRMS:Calcd.for C 8 h 12 BrN 2 (M+H):215.0175. Found: 215.0176.
[0033](2) Synthesis of 3Z-n-butenylphthalide (6): Add 20.0g (135mmol) phthalic anhydride 5 in a 500mL three-necked bottle, nitrogen protection, add 200mL a...
Embodiment 2
[0037] 2-{[1-(3,5,6-Trimethylpyrazin-2-yl)methoxy]pentyl}benzoic acid (2) and (3,5,6-trimethylpyrazin-2 - Base) the preparation of methyl-2-{1-[(3,5,6-trimethylpyrazin-2-yl) methoxy] pentyl} benzoate (3), comprises the following steps:
[0038] (1) Synthesis of 3-n-butylphthalide (NBP): In a 150mL round bottom flask, weigh 2.00g (10.6mmol) of intermediate 6, dilute and dissolve with 60mL of absolute ethanol, add 100mg of 10% Pd / C , replacing the hydrogen, and stirring overnight. After the completion of the reaction as detected by TLC, it was filtered through diatomaceous earth and concentrated to obtain 1.99 g of a colorless oily liquid with a yield of 99%. 1 HNMR (CDCl 3 , 400MHz), δ: 7.91(d, 1H, J=7.6Hz), 7.67(td, 1H, J=7.6, 1.0Hz), 7.54(t, 1H, J=7.6Hz), 7.45(d, 1H, J=7.6Hz), 5.49(dd, 1H, J=8.0, 4.8Hz), 2.08-2.00(m, 1H), 1.80-1.74(m, 1H), 1.50-1.34(m, 4H), 0.92(t ,3H,J=6.8Hz); HRMS: Calcd.for C 12 h 14 o 2 Na(M+Na):213.0886. Found: 213.0884.
[0039] (2) Synthesis of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com