Pharmaceutical preparation
A technology of pharmaceutical preparations and renin inhibitors, applied in the field of pharmaceutical preparations, can solve problems such as difficult to achieve therapeutic effects, and achieve useful therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
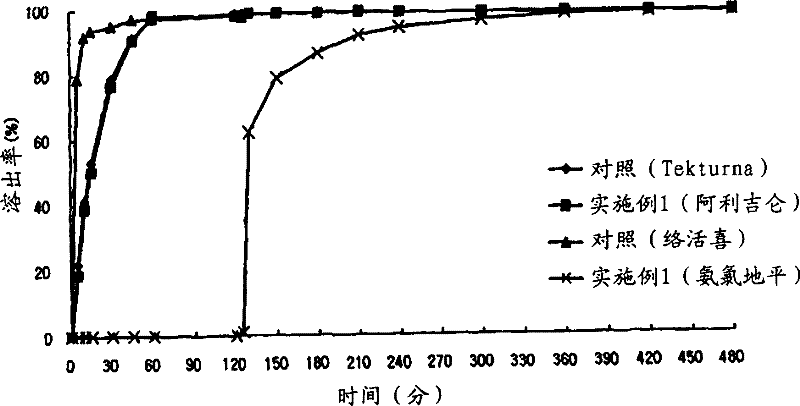
[0138] Example 1: Preparation of Aliskiren-Amlodipine Biphasic Matrix Tablets
[0139] Utilizing the composition and content in Table 1, biphasic matrix tablets were prepared according to the following method.
[0140] 1) Preparation of the prior release compartment containing Aliskiren
[0141] Aliskiren hemifumarate and microcrystalline cellulose (JRS, Vivapur.), lactose, corn starch, sodium starch glycolate (DMV, Primojel) as excipients were sieved with a No. 35 sieve, and a high-speed Mixer (Lab.Pharma Mixer P, Diosna, Germany) was used for mixing. Separately, a binding solution (5% W-W) was prepared by dissolving hydroxypropyl cellulose in water, and this binding solution was put into a high-speed mixer together with the main component mixture and kneaded. After the kneading was completed, granulation was performed using a shaker (Erweka, Germany) with a No. 18 sieve, and drying was performed at 60° C. using a hot water drier. After completion of the drying, the pre-...
Embodiment 2
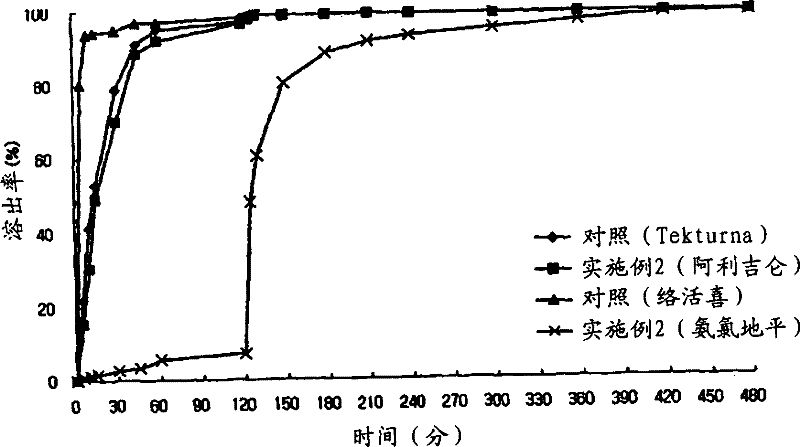
[0146] Embodiment 2: Preparation of Aliskiren-Amlodipine Multilayer Tablet
[0147] Using the composition and content in Table 1, a multi-layer tablet was prepared according to the following method.
[0148] 1) Preparation of the prior release compartment containing Aliskiren
[0149] Aliskiren hemifumarate, microcrystalline cellulose, D-mannitol, and lactose as excipients were sieved with a No. 35 sieve, and mixed with a high-speed mixer. Separately, hydroxypropyl cellulose was dissolved in water to prepare a binding solution (5% W-W), and this binding solution was kneaded together with the main ingredient mixture. After the kneading was completed, granulation was performed using a shaker with a No. 18 sieve, and drying was performed at 60° C. using a hot water drier. Once drying is complete, sieve through a No. 20 sieve again. After mixing sodium starch glycolate and adding magnesium stearate to the sieved material, a double cone mixer was used for final mixing.
[015...
Embodiment 3
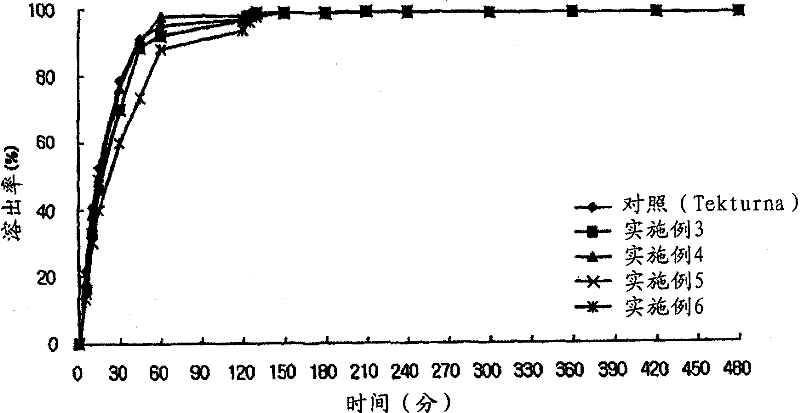
[0154] Embodiment 3: Preparation of Aliskiren-Amlodipine Capsules (Granules-Pellets)
[0155] Using the composition and content in Table 1, the capsules were prepared according to the following method.
[0156] 1) Preparation of the prior release compartment containing Aliskiren
[0157]Aliskiren hemifumarate, microcrystalline cellulose, and D-mannitol were sieved with a No. 35 sieve, and mixed with a high-speed mixer. Separately, hydroxypropyl cellulose was dissolved in water to prepare a binding solution (5% W-W), and this binding solution was kneaded together with the main ingredient mixture. After the kneading was completed, granulation was performed using a shaker with a No. 18 sieve, and drying was performed at 60° C. using a hot water drier. Once drying is complete, sieve through a No. 20 sieve again.
[0158] 2) Preparation of sustained-release compartments containing amlodipine
[0159] Amlodipine besylate, microcrystalline cellulose, and hydroxypropyl methylc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com