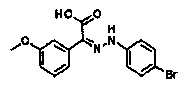
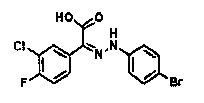
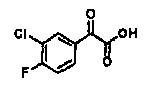
1,2,4-Triazolone Derivative
A technology of triazolone and derivatives, applied in the field of 1,2,4-triazolone derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0285] Hereinafter, reference examples, examples, and test examples are given to further describe the present invention in detail, but these do not limit the present invention and can be changed without departing from the scope of the present invention.
[0286] In Reference Examples and Examples, the "phase separator (Phase Separator)" used in the post-treatment refers to Biotage's ISOLUTE (registered trademark) phase separator. For "SNAP Cartridge KP-NH" purified by column chromatography, Biotage SNAPCartridge KP-NH was used, "SNAP Cartridge KP-Sil" was Biotage SNAPCartridge KP-Sil, and "SNAP Cartridge HP-Sil" was Biotage The company's SNAPCartridge HP-Sil, "Chromatorex NH" uses Cromatrex (registered trademark) NH manufactured by Fuji Silysia Chemical Co., Ltd. For purification using preparative thin layer chromatography (PTLC), Mercury silica gel 60F was used 254 , 20cm×20cm. Waters Sunfire prep C18 OBD, 5.0 μm, φ30×50 mm was used for “reversed phase column chromatography...
reference example P-A1
[0317] ?Reference Example P-A1: Synthesis of (3-chlorophenyl)(oxo)acetic acid
[0318] [chemical 18]
[0319]
[0320] In (3-chlorophenyl)(oxo)ethyl acetate (2.00g) in THF / MeOH solution (1:1) (48ml), add 2mol / L NaOH aqueous solution (24ml) under ice-cooling, at room temperature Stir overnight. After the solvent was distilled off under reduced pressure, a 3 mol / L HCl aqueous solution was added under ice-cooling. The precipitated solid was collected by filtration to obtain the title compound (2.00 g, colorless solid).
[0321] MS (ESI neg.) m / z : 183([M-H] - ).
[0322] The following compounds were synthesized by the same method as in Reference Example P-A1.
[0323] ?Reference Example P-A2: (3-Chloro-4-fluorophenyl)(oxo)acetic acid
[0324] [Synthesis from ethyl (3-chloro-4-fluorophenyl)(oxo)acetate]
[0325] [chemical 19]
[0326] .
reference example P-A3
[0327] ?Reference Example P-A3: Synthesis of (3-methoxyphenyl)(oxo)acetic acid
[0328] [chemical 20]
[0329]
[0330] A pyridine solution (27 ml) of 1-(3-methoxyphenyl)ethanone (8.00 g) and selenium dioxide (8.87 g) was stirred at an external temperature of 100°C for 4 hours. After standing to cool, the reaction liquid was filtered through Celait (registered trademark), diluted with EtOAc, washed with 1mol / L HCl aqueous solution and brine, and washed with Na 2 SO 4 to dry. The solvent was distilled off under reduced pressure to obtain the title compound (10.6 g, gray solid).
[0331] MS (ESI neg.) m / z : 179([M-H] - ).
[0332] The following compounds were synthesized by the same method as in Reference Example P-A3.
[0333] ?Reference Example P-A4: (4-fluoro-3-methoxyphenyl)(oxo)acetic acid
[0334] [Synthesis from 1-(4-fluoro-3-methoxyphenyl)ethanone]
[0335] [chem 21]
[0336]
[0337] MS (ESI neg.) m / z : 197([M-H] - ).
[0338] ?Reference Example P-A5: (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com