Soft nintedanib ethanesulfonate capsule and preparation method thereof
A technology of nintedanib ethanesulfonate and soft capsules, which is applied in the field of medicine, can solve the problems that the physical stability of the undisclosed suspension has an important impact, high production costs, and cumbersome processes, etc., so as to improve product stability and reduce Production cost and process requirements, the effect of reasonable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
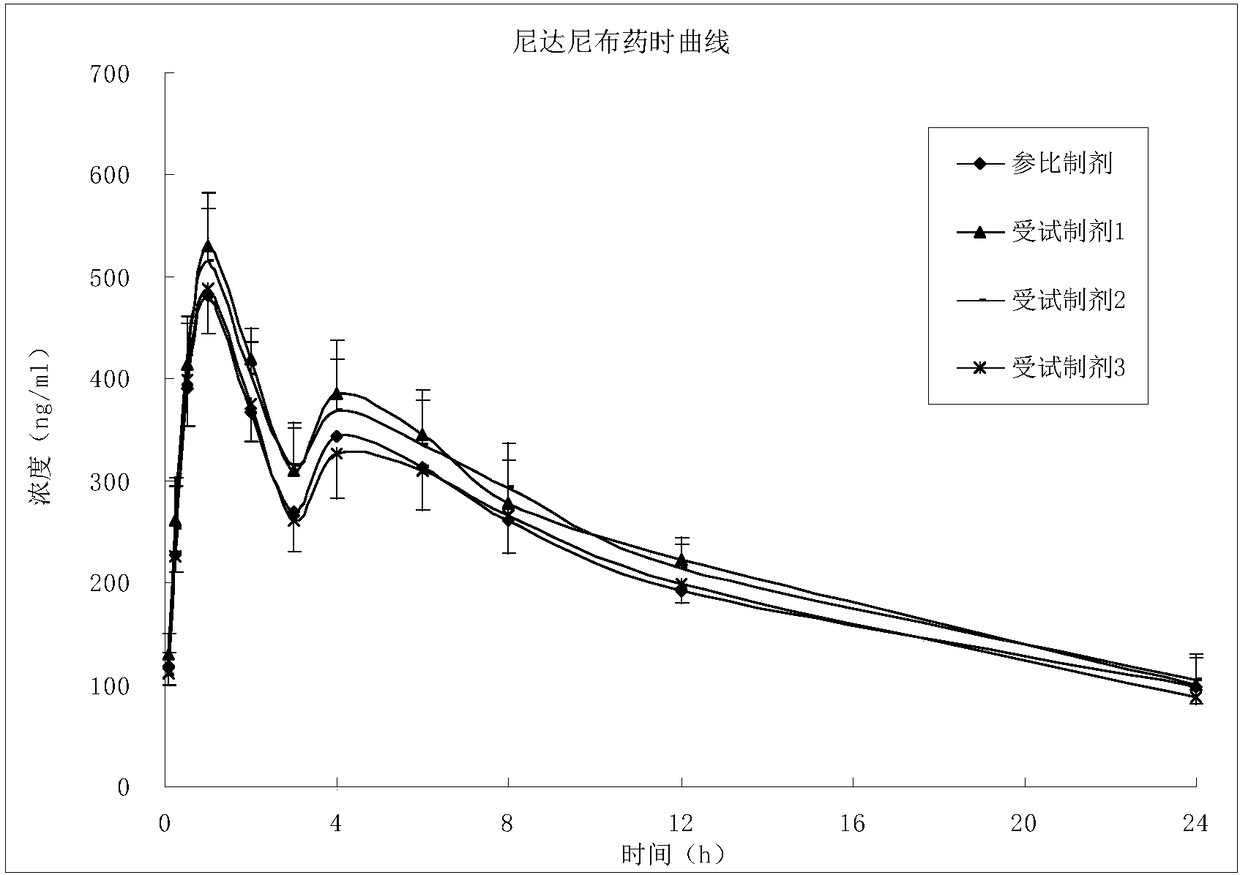
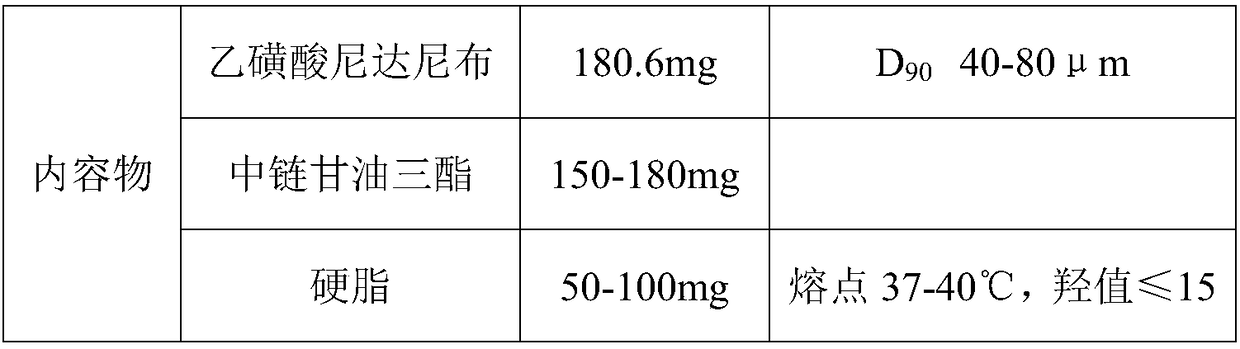
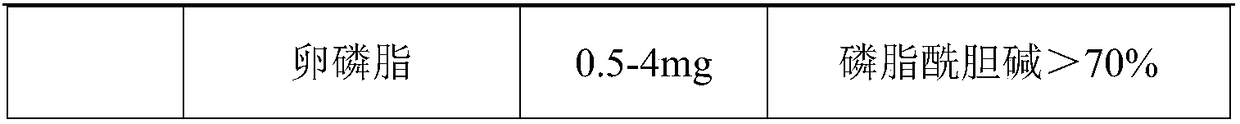
[0034] A nintedanib ethanesulfonate soft capsule, the content of which is composed of the following raw materials in parts by weight: 18.06 g of nintedanib ethanesulfonate, 15.0 g of medium-chain triglycerides, 7 g of stearin and 0.05 g of lecithin .
[0035] Preparation method: crush nintedanib ethanesulfonate through a 200-mesh sieve, measure the average particle size D90 to be 80 μm±5 μm, and set aside. Melt medium-chain triglycerides, lecithin, and stearin, then add raw materials, homogenize, and degas.
Embodiment 2
[0037] A nintedanib ethanesulfonate soft capsule, the raw and auxiliary materials used are the same as in Example 1, and the preparation method is as follows: nintedanib ethanesulfonate is pulverized through a 230-mesh sieve, and the average particle size D90 is 60 μm±5 μm. Melt medium-chain triglycerides, lecithin, and stearin, then add raw materials, homogenize, and degas.
Embodiment 3
[0039] A nintedanib ethanesulfonate soft capsule, the raw and auxiliary materials used are the same as in Example 1, and the preparation method is: nintedanib ethanesulfonate is pulverized through a 325 mesh sieve, and the average particle size D90 is 40 μm±3 μm. Melt medium-chain triglycerides, lecithin, and stearin, then add raw materials, homogenize, and degas.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com