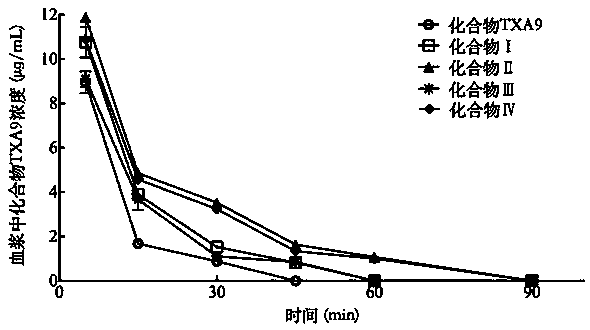
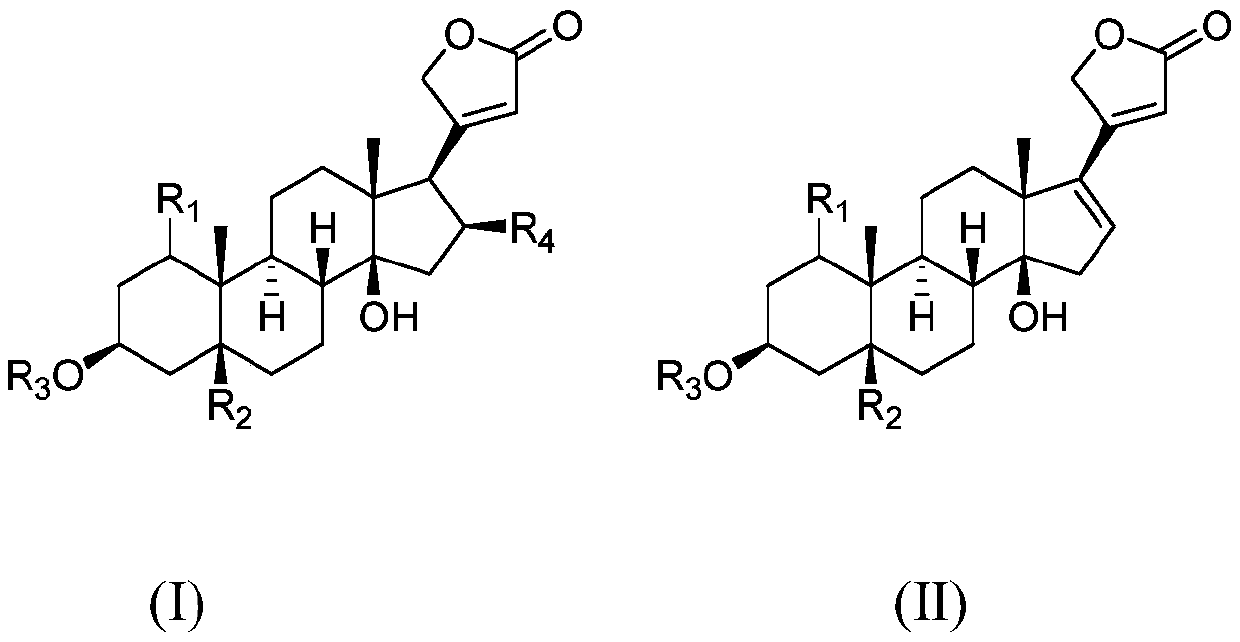
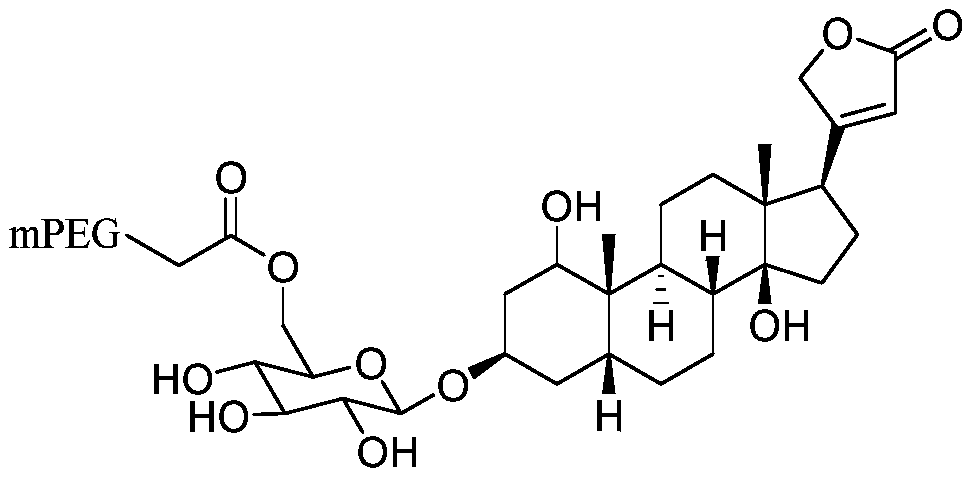
Polyethylene glycol modified cardiac glycoside compound prodrug and anti-tumor application thereof
A technology of polyethylene glycol and cardiac glycosides, which is applied in the field of medicine and can solve problems such as the application of cardiac glycosides that have not been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the preparation of compound I
[0058]
[0059] Weigh 10g mPEG 5000 , 1.56mg TEMPO, 24mg KBr (molar ratio 10:0.05:1) was dissolved in 15mL water; another 8% NaClO solution was adjusted to pH 10 with 4M HCl. The above solution was adjusted to 0° C. with an ice bath, and mixed for reaction. During the whole reaction process, the reaction temperature was kept at 0°C, and the pH of the solution was kept at 10 with 0.5M NaOH. After reacting for 5 h, ethanol was added to terminate the reaction, the pH was adjusted to 3 with 4M HCl, and then extracted three times with dichloromethane, and the dichloromethane layer was collected and washed with saturated NaCl solution, anhydrous MgSO 4 Dry for 4 hours, filter to remove the desiccant, concentrate the filtrate to a certain amount, slowly add to an appropriate amount of ice ether, crystallize overnight at 4°C, filter with suction, recrystallize the product once with anhydrous ether, weigh it after vacuum drying,...
Embodiment 2
[0062] Embodiment 2: the preparation of compound II
[0063]
[0064] Weigh 2mmol mPEG 5000 , add 200mL of toluene to dissolve, heat and reflux in an oil bath at 120°C for 2h, evaporate the solvent to dryness under reduced pressure at 75°C, then add 150mL of anhydrous dichloromethane into the round-bottomed flask to dissolve, weigh succinic anhydride (20mmol), pyridine (0.4 mmol) was added to the above system, after the reaction vessel was airtight, stirred in an oil bath at 37°C for 24h, and was detected by TLC (I 2 Color development) after the completion of the reaction, evaporate the solvent to dryness under reduced pressure, add saturated NaHCO 3 Dissolve, adjust the pH to 2 with concentrated hydrochloric acid, extract 3 times with dichloromethane, collect the dichloromethane layer, and wash with saturated NaCl solution, anhydrous MgSO 4 Dry for 4 hours, filter to remove the desiccant, concentrate the filtrate to a certain amount, slowly add to an appropriate amount o...
Embodiment 3
[0067] Embodiment 3: the preparation of compound III
[0068]
[0069] Accurately weigh mPEG (1.00mmol), phenyl p-nitrochloroformate (5.00mmol), and DMAP (2.00mmol) in a 100mL round bottom flask, add 60mL of anhydrous dichloromethane, and react at 25°C for 12h. TLC detected that the reaction was complete, and the reaction solution was extracted three times with an equal volume of 10% citric acid aqueous solution and saturated sodium chloride aqueous solution three times, and the organic layer was dried with anhydrous sodium sulfate for 4 hours, filtered and concentrated, and the product was purified by silica gel column chromatography , a white powdery solid, ie, mPEG-pNP, was obtained with a yield of 82%. 1 H NMR (600MHz, CDCl 3 )δ8.29(d,J=9.2Hz,2H),7.40(d,J=9.2Hz,2H),4.45-4.44(m,2H),3.82-3.81(m,2H),3.65(br.s ),3.38(s,3H).
[0070]Accurately weigh the mPEG-pNP (0.19mmol) prepared above, add 30mL of toluene, stir, reflux at 115°C for 2h, evaporate the solvent under reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com