Piperidine carbamic acid ester derivative and application thereof
A methyl and group technology, applied in the field of pharmacy, can solve the problems of low bioavailability and limited clinical application prospects, achieve good oral bioavailability, and optimize the effect of blood sugar in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
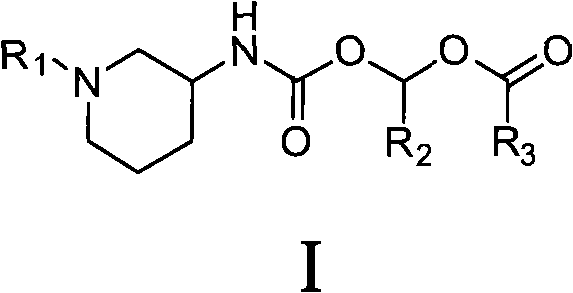
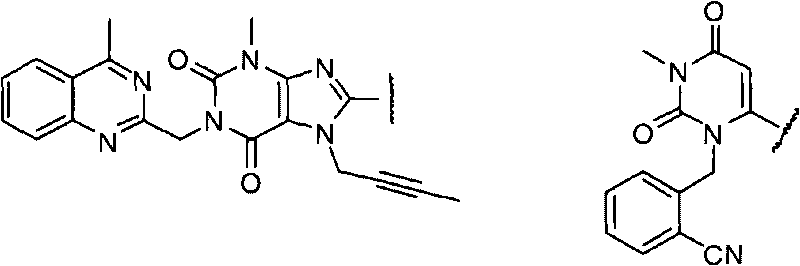
[0051] Example 1 Isobutyric acid-1-((R)-1-(7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolinyl-2-yl) Methyl)-2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)piperidin-3-ylcarbamoyloxy)ethyl ester (compound 1) preparation
[0052] (R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolin-2-yl )methyl)-1H-purine-2,6(3H,7H)-diketone (Linagliptin, 1.46g, 3.1mmol) was dissolved in 40ml of anhydrous N,N-dimethylformamide solution, and triethylamine was added (1.3ml, 9.3mmol), under the protection of nitrogen, cooled to 0°C in an ice bath, slowly added 1-(isobutyryloxy)ethyl chloroformate (0.60g, 3.1mmol), and warmed to room temperature after the addition, The reaction was stirred for 6 hours. After the reaction was completed, 80 ml of ice water was added, extracted with dichloromethane (100 ml×3), washed with saturated brine, and dried overnight over anhydrous sodium sulfate. The desiccant was filtered off, concentrated under reduced pressure, and the residue was puri...
Embodiment 2
[0053] Example 2 pivalic acid-(R)-(1-(7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolin-2-yl)methyl) Preparation of -2,6-dioxo-2,3,6,7-tetrahydro-1H-purin-8-yl)piperidin-3-ylcarbamoyloxy)methyl ester (compound 2)
[0054] Referring to the method for preparing compound 1 in Example 1, (R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-methyl-1-((4 -Methylquinazolin-2-yl)methyl)-1H-purine-2,6(3H,7H)-dione (1.46g, 3.1mmol) and (pivaloyloxy)methyl chloroformate ( 0.60 g, 3.1 mmol) to obtain 1.08 g of powdery solid, yield 55.4%. 1 HNMR (CDCl 3 ), δ(ppm): 1.15-1.32(m, 10H), 1.53-1.94(m, 6H), 2.70-2.91(m, 5H), 2.94-3.10(m, 1H), 3.41(s, 3H), 3.54-3.73(m, 2H), 4.92(m, 2H), 5.34(s, 2H), 5.76(s, 2H), 7.70(m, 1H), 7.82(d, J=7.8Hz, 1H), 7.94 (m, 1H), 8.26 (d, J=7.8Hz, 1H). ESI-MS: 631.3 (M+1).
Embodiment 3
[0055] Example 3 Isobutyric acid-1-((R)-(1-(3-(2-cyanobenzyl)-1-methyl-2,6-dioxo-1,2,3,6- Preparation of tetrahydro-pyrimidin-4-yl)piperidin-3-ylcarbamoyloxy)ethyl ester (compound 3)
[0056] Referring to the method for preparing compound 1 in Example 1, (R)-2-((6-(3-aminopiperidin-1-yl)-3-methyl-2,4-oxo-3,4-di Hydropyrimidin-1(2H)-yl)methyl)benzocyanide (Alogliptin, 1.05 g, 3.1 mmol) was reacted with 1-(isobutyryloxy)ethyl chloroformate (0.60 g, 3.1 mmol) to give 0.89g powdery solid, yield 57.4%. 1 HNMR (CDCl 3 ), δ (ppm): 1.16 (d, J=8.2Hz, 6H), 1.40-1.69 (m, 5H), 1.85-2.21 (m, 2H), 2.41-2.73 (m, 3H), 2.90-3.46 ( m, 7H), 5.14-5.35(m, 3H), 6.80(m, 1H), 7.28(d, J=7.8Hz, 1H), 7.40(m, 1H), 7.61(m, 1H), 7.68(d , J=7.1Hz, 1H). ES-MS: 498.2 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com