Arsenic-trioxide-carrying pH-responsive mesoporous silica nanoparticle and preparation method thereof
A technology of mesoporous silica and arsenic trioxide, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of lack of specificity in ATO distribution, limited application, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of nanoparticle
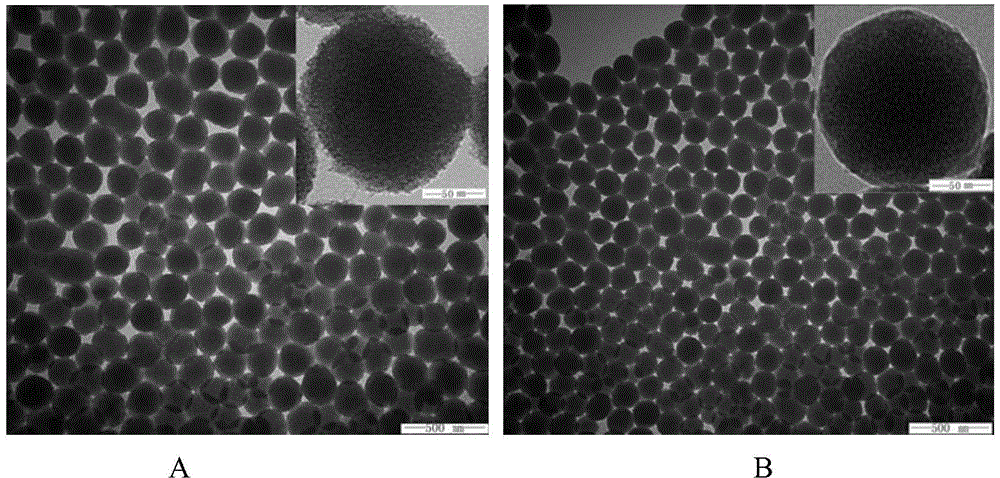
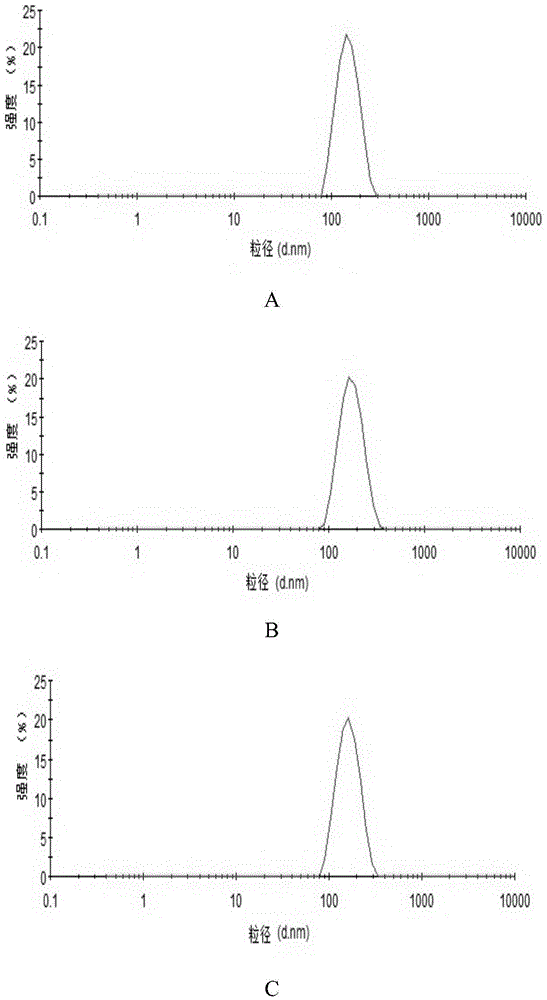
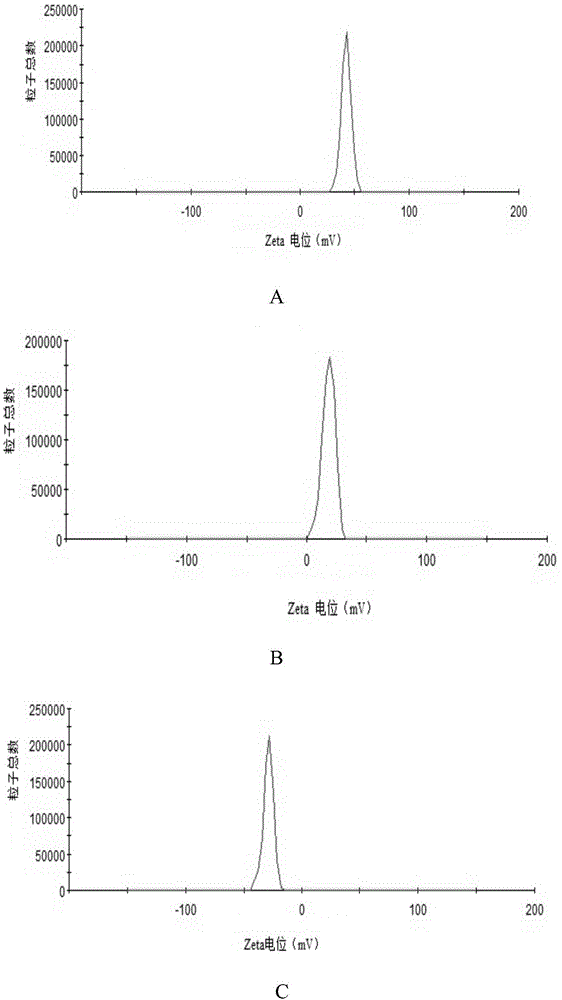
[0026] NH 2- Preparation of MSNs: One-step preparation of aminated mesoporous silica by co-precipitation method. 0.3g CTAB was dissolved in ultrapure water, and an appropriate amount of 2mol / L NaOH solution was added to adjust the pH to about 11.5. After magnetic stirring at 80°C for 0.5 h, the mixed solution containing 1 mL of TEOS and 0.5 mL of APTES was added dropwise to the CTAB solution, reacted for 2 h, left to mature for 6 h, centrifuged at 20 000 r / min for 30 min, acidic ethanol (absolute ethanol and Hydrochloric acid (volume ratio 10:1) was washed three times to remove the template agent CTAB. Wash three times with ultrapure water, centrifuge, and freeze-dry to obtain NH 2 -MSNs.
[0027] Preparation of PAA-ATO-MSNs: Accurately weigh 40 mg of MSNs freeze-dried powder, add to 10 mL of 1 mg / mL ATO solution, and stir for 24 hours. 20 000r / min high-speed centrifugation for 30 minutes to remove free ATO, washed th...
Embodiment 2
[0038] Example 2: Determination of Encapsulation Efficiency and Drug Loading Capacity
[0039] Instrument working conditions
[0040] RF power: 1150W; plasma flow: 50L / min; auxiliary gas flow: 0.5L / min; atomizer flow: 0.3L / min; pump speed: 50r / min; instrument stabilization delay: 5s; cleaning time: 30s ; Carrier gas: argon (purity 99.99%); analytical line: 189nm.
[0041] Linear relationship investigation
[0042] Precisely pipette an appropriate amount of arsenic standard solution into a 100mL volumetric flask, dilute to the mark with dilute nitric acid, and obtain a series of arsenic standard solutions with mass fractions of 0, 0.25, 0.5, 1.0, 2.0, 4.0, 8.0 mg / L. Measure the emission intensity of each element in the standard blank solution and each standard solution at 189nm, take the emission intensity (Y) as the ordinate, and the concentration (C) as the abscissa, draw a standard curve and get the correlation coefficient by the software iTAVA: Y= 1847.2C+19.606 (r=0.999...
Embodiment 3
[0048] Implementation 3: In vitro drug release study
[0049] PBS (5.0, 6.0, 7.4) solutions with different pH were selected as the release medium to investigate the release characteristics of ATO in the drug-loaded nanoparticles. ATO 0.5mg) was dissolved or dispersed with 2mL release medium, placed in a pre-treated dialysis bag, sealed after removing air bubbles, placed in 100mL release medium, and shaken in a constant temperature water bath (75r / min) at (37±0.5)°C, Accurately sample 2 mL at 0.1, 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 12, and 24 hours, and immediately add an equal amount of fresh release medium at the same temperature and pH, and the samples are filtered through a 0.22 μm microporous filter. Membrane filtration, measure the drug content in the release medium after taking the continued filtrate dilution, calculate the cumulative drug release rate (Q%), and draw the drug release curve as Figure 9 . It can be seen from the figure that there is no significant dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com