Heterodimer multifunctional prodrug based on camptothecin and preparation method and application of heterodimer multifunctional prodrug
A technology of heterodimer and camptothecin is applied in the field of heterodimer multifunctional prodrugs and achieves the effects of efficient encapsulation, simple synthesis and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
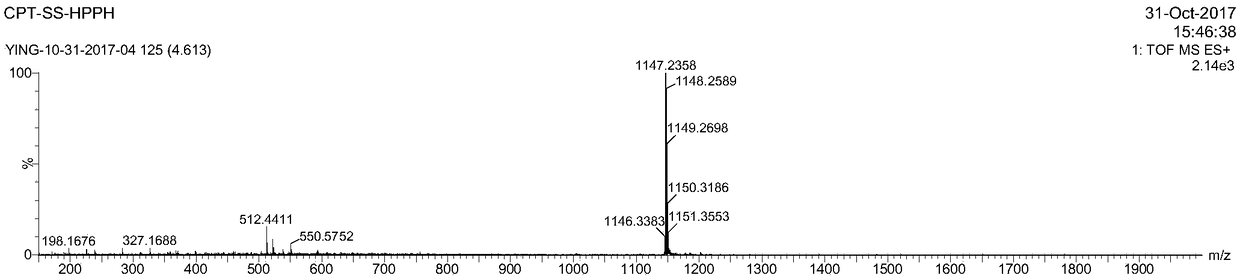
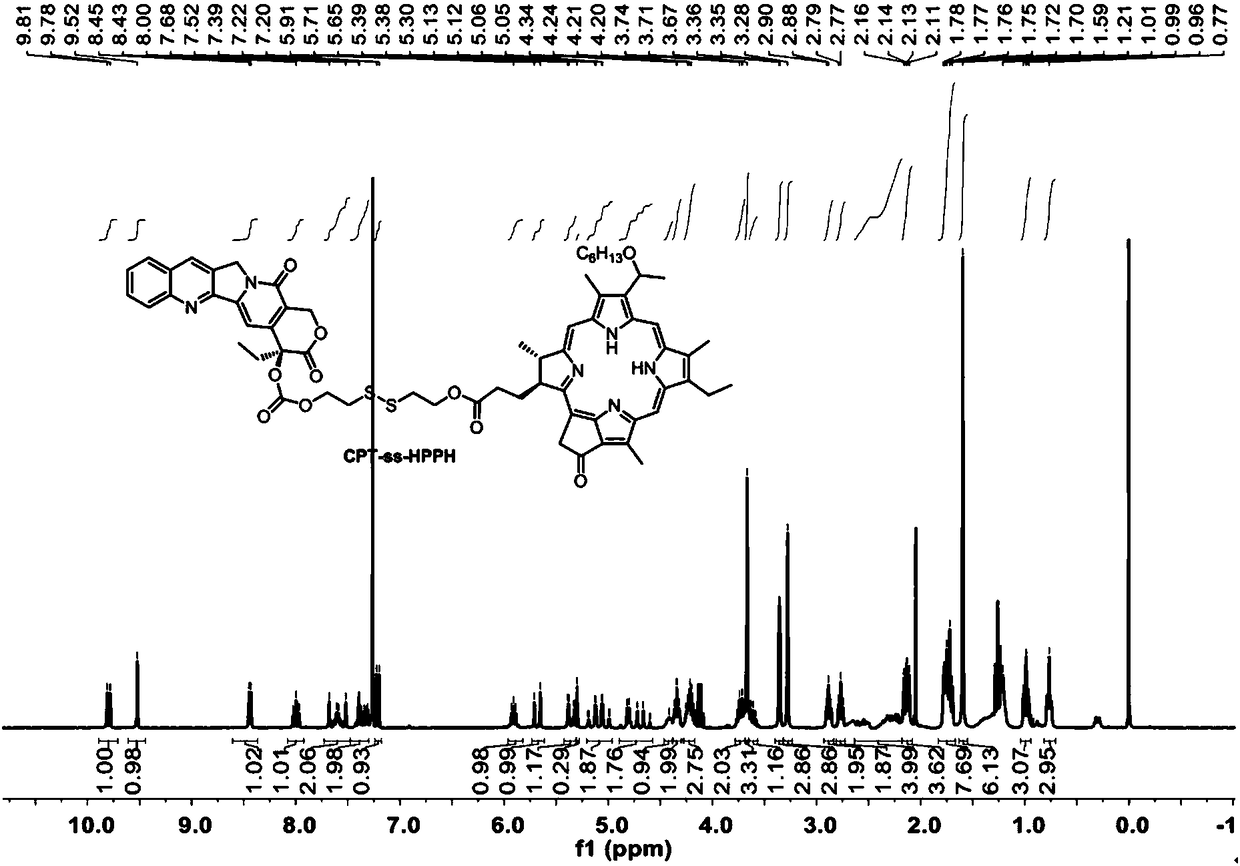
[0067] Embodiment 1: the preparation of CPT-ss-HPPH
[0068] DMAP (1.15 mg, 9.42 μmol), HPPH (30 mg, 4.71 μmol) and CPT-ss-OH (51.0 mg, 94.3 μmol) were mixed in 20 mL of dichloromethane. A solution of 5 mL of EDC HCl (18.1 mg, 94.3 μmol) in dichloromethane was added dropwise with stirring. After stirring at room temperature for 24 hours, complete reaction of HPPH was confirmed by thin layer chromatography (TLC). The mixture solution was concentrated using a rotary evaporator and then purified by flash chromatography (Teledyne ISCOCombiFlash) using a gradient of ethyl acetate and hexanes as eluents. Yield 39.9 mg (74%). ESI-MS m / z (M + )calcd 1146.46,found 1147.24(M+H + ). 1 H NMR (300MHz, CDCl 3 )δ9.80(d, J=8.9Hz, 1H), 9.52(s, 1H), 8.44(d, J=5.7Hz, 1H), 8.00(t, J=8.7Hz, 1H), 7.69–7.51( m,2H),7.42–7.28(m,2H),7.21(d,J=7.5Hz,1H),5.91(p,J=6.5Hz,1H),5.68(d,J=17.2Hz,1H), 5.38–5.3(m,1H),5.09(qd,J=20.0,3.0Hz,2H),4.81(d,J=6.0Hz,1H),4.69(dd,J=17.3,1.2Hz,1H),4.44 –4.38(m,1H),4.4...
Embodiment 2
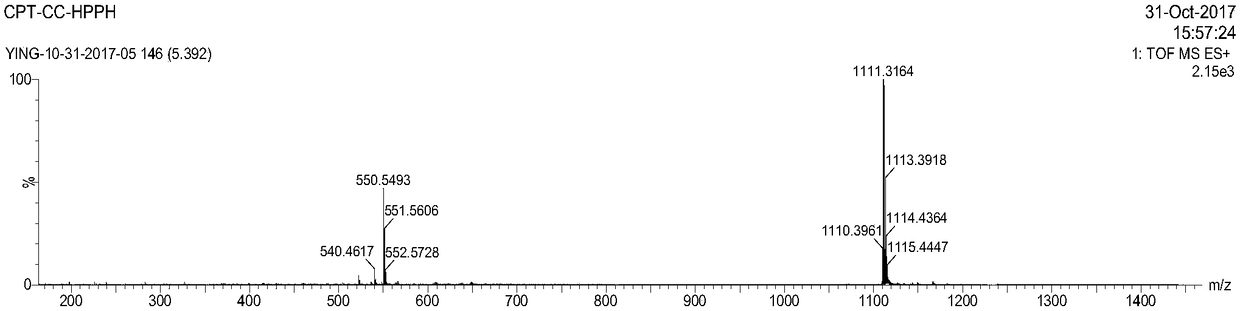
[0070] Embodiment 2: Preparation of CPT-cc-HPPH
[0071]DMAP (0.58 mg, 4.75 μmol), HPPH (15 mg, 23.5) and CPT-cc-OH (23.8 mgmg, 47.1 μmol) were mixed in 20 mL of dichloromethane. A solution of 5 mL of EDC HCl (9.1 mg, 47.1 μmol) in dichloromethane was added dropwise with stirring. After stirring at room temperature for 24 hours, complete reaction of HPPH was confirmed by thin layer chromatography (TLC). The mixture solution was concentrated using a rotary evaporator and then purified by flash chromatography (Teledyne ISCOCombiFlash) using a gradient of ethyl acetate and hexanes as eluents. Yield: 22.5 mg (86%).ESI-MS m / z (M + )calcd 1110.55,found 1111.32(M+H + ). 1 H NMR (300MHz, CDCl 3 )δ9.78(d,J=4.1Hz,1H),9.52(s,2H),8.50(s,1H),8.19–7.95(m,4H),7.79–7.59(m,2H),7.57–7.45 (m,2H),7.27(s,2H),5.98–5.83(m,2H),5.68(d,J=17.3Hz,2H),5.37(dd,J=17.1,1.1Hz,2H),5.28( d,J=8.9Hz,1H),5.20(s,2H),5.17–5.02(m,3H),4.46(q,J=7.4Hz,1H),4.28(d,J=8.3Hz,1H), 4.11–3.98(m,2H),3.99–3.81(m,2H),3.79–...
Embodiment 3
[0073] Example 3: Generation of Reactive Oxygen Species (ROS) by HPPH and CPT-ss-HPPH
[0074] The ROS generation of HPPH and CPT-ss-HPPH prepared in Example 1 was measured by using anthracene-9,10-dipropionic acid (ADPA) as ROS quencher. Briefly, ADPA was dissolved in DMSO and diluted to have a UV-Vis absorbance of 1.1 at 410 nm. HPPH or CPT-ss-HPPH in 1 mM DMSO was then added to a final concentration of 10 μM. The obtained solution was irradiated with a 671 nm laser at an intensity of 100 mW for 7 minutes. At each minute, the UV-Vis absorbance of the solution was recorded by a UV3100PC spectrophotometer (VWR, Radnor, PA). The obtained results were analyzed by Origin 8.
[0075] like Figure 6 As shown, we found that the absorbance of HPPH and CPT-ss-HPPH decreased continuously with the increase of irradiation time. Notably, the absorbance of CPT-ss-HPPH decreased faster than that of HPPH, indicating that CPT-ss-HPPH was able to generate more ROS ( Figure 6 a-c). Furt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com